Figures & data
A Depicted is the clinical course of ducks during acute phase after infection with HPAIV H5N8 viruses belonging either to clade 2.3.4.4A (DE14-H5N8A) (upper panel) or clade 2.3.4.4B (DE16-H5N8B) (lower panel). Adult pekin ducks were inoculated oronasally (o.n.) (left panel) and 1-week old pekin- or muskovy ducks were inoculated intramuscularly (i.m.). In addition, clinical course of sentinel ducks (red symbols) and sentinel chickens (graphs on the right) kept in direct contact to adult ducks is shown. Numbers within the figures give the clinical score for individual groups according to the IVPI scoring systemCitation59. B Oro-pharyngeal (upper panel) and cloacal swabs (lower panel) were taken from adult ducks and chickens (sentinel to DE16 H5N8B infected ducks) at indicated days post infection (dpi) or days post contact (dpc) and tested by RT-qPCR. Individual results of detected RNA copy numbers are given as virus equivalents (VE), calculated by using a set of standards applied in each run, with the blue line indicating the threshold. Besides individual results of inoculated birds (open circles), arithmetic mean and s.d. of infected virus-positive samples (red dots) are shown, as well as sentinel birds (triangles). Numbers within the figures provide the number of virus positive -and the number of total tested infected animals at each time point
Semiquantitative AIV antigen detection in avian species by IHC
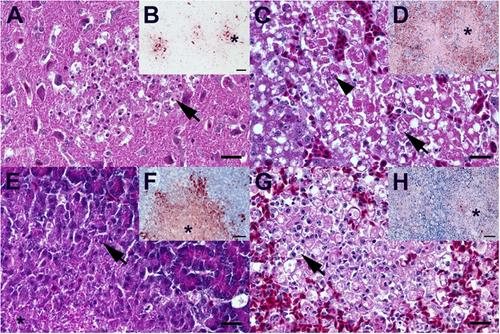
A Duck, 8 dpi, brain. Moderate, subacute necrotizing polioencephalitis with infiltration of phagocytic microglia (arrow). B Duck, 8 dpi, brain. Multiple foci (star) of influenza A nucleoprotein-immunoreactive neuronal and glia cells. C Duck, 5 dpi, liver. Severe, acute, hepatic necrosis (necrotizing hepatitis) characterized by cytoplasmic hypereosinophilia and vacuolation (arrow), membraneous rupture and nuclear pyknosis, karyorrhexis (arrowhead) and loss. D Duck, 5 dpi, liver. Influenza A virus-nucleoprotein-immunoreactive hepatocytes are typically located at the border of the coalescing necrotizing lesions, whereas only faintly immunoreactive cellular debris is present in the lesion centers (star). E Duck, 5 dpi, pancreas. Moderate, acute pancreatic liquefactive necrosis (necrotizing pancreatitis) characterized by granular eosinophilic debris (star) and scant nuclear pyknosis, karyorrhexis (arrow) and loss. F Duck, 5 dpi, pancreas. Influenza A virus-nucleoprotein-immunoreactive exocrine pancreatocytes are typically located at the border of the multifocal necrotizing lesions, whereas only faintly immunoreactive cellular debris is present in the lesion centers (star). G Duck, 4 dpi, spleen. Severe, diffuse, lymphatic depletion and marked tingible body macrophage (arrow) hyperplasia within the white pulp surrounded by hyperemic red pulp. H Duck, 4 dpi, spleen. Oligo- to multifocal discrete influenza A virus-nucleoprotein-immunoreactive cells interpreted as macrophages/dendritic cells, endothelia and faintly immunoreactive debris within the depleted white pulp (star) and less frequently in the hyperemic red pulp. A, C, E, G Hematoxylin eosin, bar = 20 µm. B, D, F, H Influenza A virus-nucleoprotein immunohistochemistry, avidin-biotin-peroxidase complex method with a polyclonal rabbit anti- influenza A FPV/Rostock/34-virus-nucleoprotein antiserum (diluted 1:750)Citation65 3-amino-9-ethyl-carbazol as chromogen and hematoxylin counterstain, bar = 50 µm
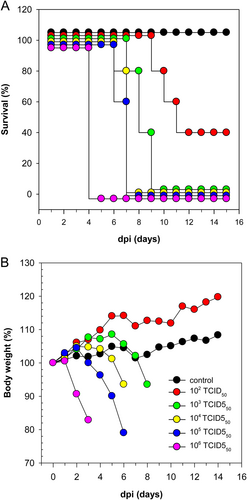
A Survival percentage and B change in body weight of 4-week old BALB/c mice infected intranasally with five different infectious doses: 102 to 106 TCID50 given in relation to the day post inoculation (dpi). Values generated from control animals are depicted in black, dosage group 102 TCID50 in red, 103 TCID50 in green, 104 TCID50 in yellow, 105 TCID50 in blue, 106 TCID50 in purple
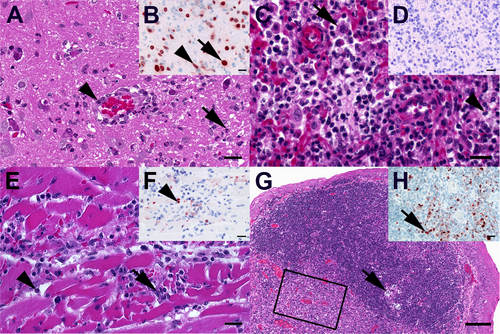
A Mouse, medulla oblongata. Moderate, acute, multifocal, necrotizing encephalitis with few neutrophils (arrow), microgliosis and activated hypertrophic vascular endothelium (arrowhead). B Mouse, medulla oblongata. Multifocal influenza A nucleoprotein in neurons (arrow) and glial cells (arrowhead). C Mouse, lung. Moderate, subacute, multifocal, lymphohistiocytic, interstitial pneumonia with alveolar histiocytosis (arrow), and variable amounts of intraalveolar neutrophils (arrowhead), and alveolar edema. D Mouse, lung. No influenza A virus-nucleoprotein-immunoreactive cells in lung section. E Mouse, heart. Mild, acute, multifocal, necrotizing myocarditis with infiltrating macrophages (arrow) and neutrophils (arrowhead). F Mouse, heart. Oligofocal influenza A virus-nucleoprotein-positive round cells (arrowhead) and cellular debris. G Ferret, tonsilla palatina. Prominent follicular germ center with tingible body macrophages (arrow) and an excentric, polar zone of differentiated lymphocytes H Ferret, tonsilla palatina. Boxed area in (G) Oligofocal round cells (macrophages) with influenza A virus nucleoprotein-positive nuclei (arrow) mainly in interfollicular areas. A, C, E, Hematoxylin eosin, bar = 20 µm. G Hematoxylin eosin, bar = 100 µm. B, D, F, H Influenza A virus-nucleoprotein immunohistochemistry, avidin-biotin-peroxidase complex method with with a polyclonal rabbit anti- influenza A FPV/Rostock/34-virus-nucleoprotein antiserum (diluted 1:750)Citation65 3-amino-9-ethyl-carbazol as chromogen and hematoxylin counterstain, bar = 20 µm
A Change in body weight data of inoculated ferrets (open circle) and contact ferrets (filled diamond), and B change in body temperature of intranasally infected and contact ferrets. C Tabular summary of viral RNA loads detected from nasal washing samples as expressed by genome copy number per µl; the only sample from which virus could be re-isolated is highlighted in red. D Tabular summary of hemagglutination inhibition titers in sera from infected and sentinel animals
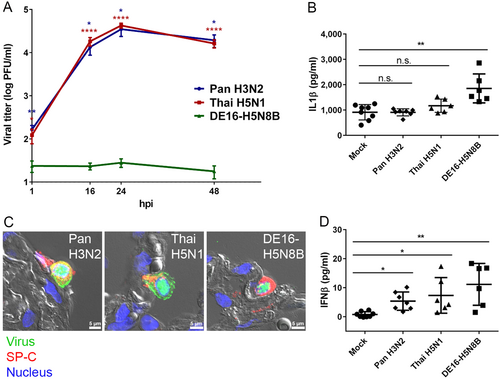
A Tumor-free human lung tissue was infected with 2 × 105 PFU of IAV Pan H3N2, Thai H5N1 or DE16-H5N8B. At 1, 16, 24 and 48 hpi, supernatants of infected lung tissue were harvested and viral titers were determined by standard plaque titration assay. Mean values and standard errors of the means (SEM) from four independent experiments, each done in triplicate, are shown. Asterisks indicate significant differences between IAV strains Pan H3N2 (blue) or Thai H5N1 (red) compared to DE16-H5N8B at each indicated time point (Mann–Whitney U test; *p ≤ 0.05; **p ≤ 0.01; ****p ≤ 0.0001). B, D Tumor-free human lung tissue was infected with 1 × 106 PFU of IAV Pan H3N2, Thai H5N1 or DE16-H5N8B. Aliquots of infected lung culture supernatants 24 hpi were analyzed for the concentrations of IL1β (B) or IFNβ (D) in pg/ml by commercially available ELISA sets. Data points from three independent experiments done in biological duplicates are shown individually with mean values and standard deviation, respectively. Significance values between virus and mock infected samples are indicated by asterisks (one-way-Anova with Dunn’s multiple comparisons test; *p ≤ 0.05; **p ≤ 0.01). C Tumor-free human lung tissue was infected with 1 × 106 PFU of IAV Pan H3N2, Thai H5N1 or DE16-H5N8B. Virus (green) was typically detected in alveolar epithelial type II cells indicated by pro-SP-C (red). Lung structure was visualized by differential interference contrast (grey) and nuclei were counterstained using DAPI (blue). Scale bar 5 µm (panel C)