Figures & data
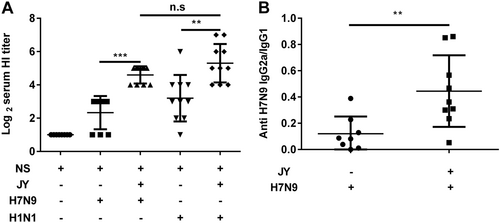
Mice were immunized twice (1-week interval) with the H7N9 or H1N1 influenza vaccine with or without JY adjuvant. The HI titers in serum were determined by the HI assay with virus H7N9 (A/shanghai/02/2013) or H1N1 (A/California/07/2009). The subclasses of the anti-HA IgG (IgG2a and IgG1) in serum were detected by ELISA. The data are shown as the geometric mean of mice in each group with their corresponding SD on a log 2 scale, and the results were compared using Student’s t-test. Differences with a P-value < 0.05 were considered statistically significant. Significant differences between groups are indicated as **P < 0.01, ***P < 0.001, or n.s. no significant difference
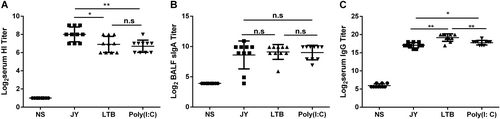
Mice were intranasally immunized with 4.5 μg HA twice (3-week interval) and with different adjuvants. Serum and BALF were collected at day 21 after the last immunization, and the titers of HI and IgG in serum and of sIgA in BALF were detected. The data are shown as the geometric mean of mice in each group with the corresponding SD on a log 2 scale, and the results were compared using Student’s t-test. Differences with a P-value < 0.05 were considered statistically significant. Significant differences between groups are indicated as *P < 0.05, **P < 0.01, or n.s. no significant difference
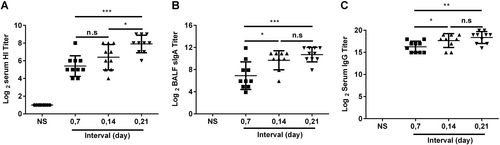
BALB/c mice were randomly divided into four groups and intranasally immunized with the JY-adjuvanted H7N9 nasal spray vaccine containing 4.5 μg HA at 7-day, 14-day, and 21-day intervals or an NS control. Three weeks after the last immunization, serum and BALF were collected. The titers of HI and anti-HA IgG in serum and of sIgA in BALF were detected using the HI assay or ELISA. The data are shown as the geometric mean of all mice in each group with the corresponding SD on a log 2 scale, and the results were compared using Student’s t-test. Differences with a P-value < 0.05 were considered statistically significant. Significant differences between groups are indicated as *P < 0.05, **P < 0.01, ***P < 0.001, or n.s. no significant difference
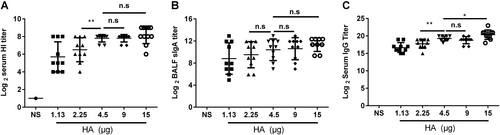
BALB/c mice were randomly divided into five groups and intranasally immunized with the JY-adjuvanted nasal spray H7N9 vaccine with a 21-day interval but with different HA contents: 15, 9, 4.5, 2.25, or 1.13 μg. An NS control group was also included. Serum and BALF were collected 21 days after the last immunization. The titers of HI and anti-HA IgG in serum and of sIgA in BALF were detected using the HI assay or ELISA. The data are shown as the geometric mean of all mice in each group with the corresponding SD on a log 2 scale, and the results were compared using Student’s t-test. Differences with a P-value < 0.05 were considered statistically significant. Significant differences between groups are indicated as *P < 0.05, **P < 0.01, or n.s. no significant difference
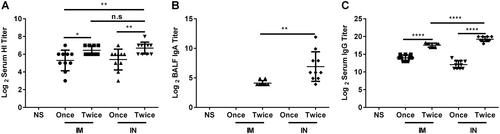
Mice were intranasally or intramuscularly immunized with vaccines once or twice (3-week interval). Serum and BALF were collected 21 days after the last immunization, and then the titers of HI and anti-HA IgG in serum and of sIgA in BALF were determined. The data are shown as the geometric mean of all mice in each group with the corresponding SD on a log 2 scale, and the results were compared using Student’s t-test. Differences with a P-value < 0.05 were considered statistically significant. Significant differences between groups are indicated as *P < 0.05, **P < 0.01, ****P < 0.0001, or n.s. no significant difference. IN intranasal administration, IM intramuscular administration
Intranasal immunization design in the different groups
