Figures & data
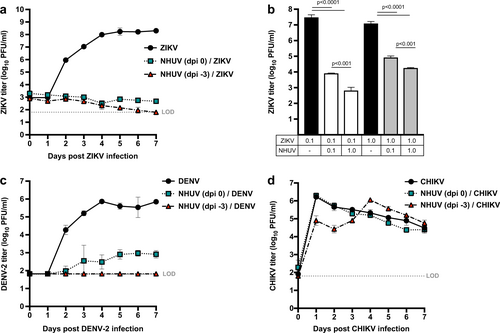
ZIKV (a) titers of C6/36 cells superinfected with NHUV (MOI 5) at −3 or 0 dpi prior to ZIKV infection (MOI 0.1). b ZIKV titers at 7 dpi from cells co-inoculated with NHUV or ZIKV at varying MOI combinations (MOI = 0.1 or 1.0). DENV-2 (MOI 0.1) (c) or CHIKV (MOI 0.1) (d) titers of C6/36 cells superinfected with NHUV (MOI 5) at −3 or 0 dpi. Inoculations for all groups were performed in triplicate. The limit of detection (LOD) for ZIKV, DENV-2, or CHIKV was 1.8 log10 PFU/ml culture supernatant
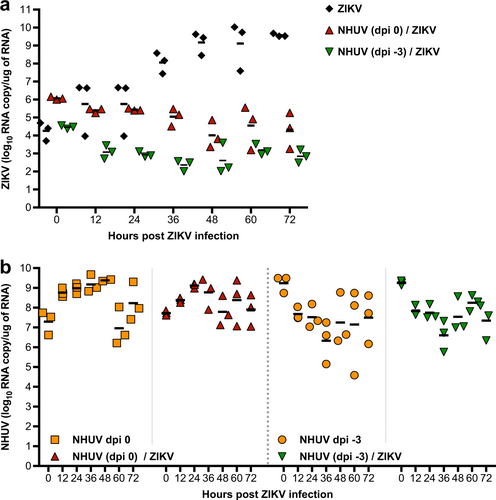
a Intracellular ZIKV RNA copy number in C6/36 cells superinfected with NHUV (MOI 5.0) at −3 dpi or concurrently inoculated with NHUV (MOI 5.0) and ZIKV (MOI 0.1). b The same NHUV/ZIKV groups assayed for ZIKV copy number were also assayed from NHUV and compared to intracellular NHUV RNA copy number in C6/36 cells inoculated with NHUV only at similar time points. For all groups, RNA was extracted from harvested cells and the copy number for NHUV or ZIKV was determined by qRT-PCR. Infections for all groups were performed in triplicate. Each point represents total extracted RNA from a single well
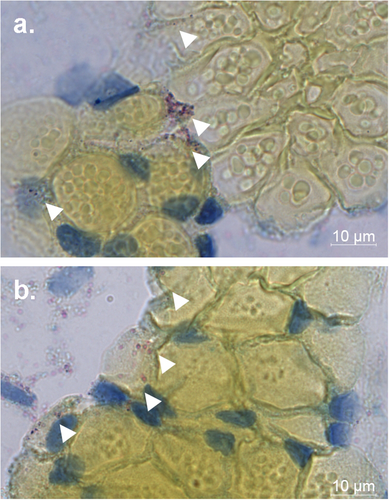
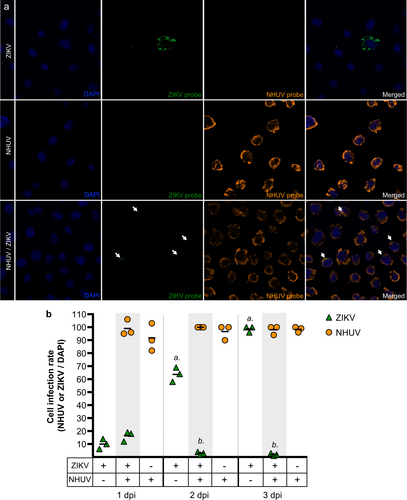
a ISH of C6/36 cells co-inoculated with ZIKV and NHUV or singly inoculated with NHUV and ZIKV and imaged at 1 dpi. b Quantification of DAPI stained cells that were also positive by ISH for NHUV, ZIKV, or NHUV and ZIKV RNA assessed through 1–3 dpi. The nuclei were stained by DAPI. Comparisons where the p-values are lower than 0.05 were observed for ZIKV positive cells in groups inoculated with ZIKV only or NHUV/ZIKV and is denoted by a and b, where a is significantly different from b
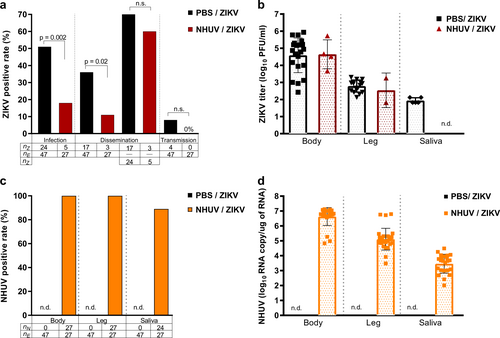
2–4 day-old Ae. aegypti were IT inoculated with NHUV or PBS and per orally exposed to a ZIKV infectious blood meal at 6 days post IT inoculation. a Infection, dissemination, and transmission rates for ZIKV. Infection rates were determined as the number of ZIKV positive bodies (nZ) as a function of blood fed mosquitoes (ne). Dissemination rates were calculated as the number of ZIKV positive legs (nz) per exposed mosquitoes (ne) as well as the number of ZIKV positive legs from ZIKV positive bodies. Transmission rates were calculated as the percentage of ZIKV exposed mosquitoes (ne) that were also positive for virus in saliva (nz). b ZIKV titers for bodies, legs and salivary expectorants. c Percent of bodies, legs, and salivary expectorants positive for NHUV RNA. The percentage of ZIKV positive bodies determined as the number of NHUV RNA positive bodies (nN) as a function of inoculated mosquitoes (nE). The percent of infected legs was calculated as the number of NHUV RNA positive legs (nN) as a function of inoculated mosquitoes (nE). The percent of positive saliva expectorants was calculated as the number of NHUV-inoculated mosquitoes (nN) that were also positive for NHUV RNA in saliva (nE). Not detected (n.d.) d NHUV RNA copy number detected for bodies, legs, and salivary expectorants. Not detected (n.d.)
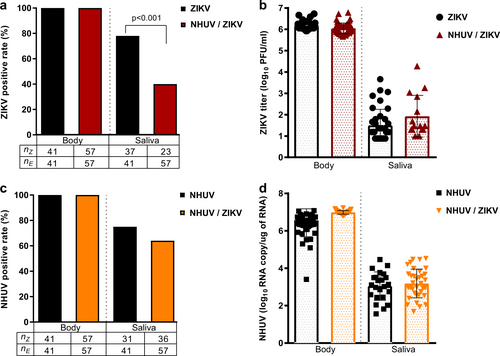
Ae. aegypti IT inoculated with NHUV, ZIKV, or NHUV and ZIKV. a Percent of bodies positive for ZIKV and percent of saliva expectorants positive for ZIKV for mosquitoes IT inoculated with ZIKV or NHUV and ZIKV. b ZIKV titers of mosquito bodies or salivary expectorants for mosquitoes IT inoculated with ZIKV or NHUV and ZIKV. c NHUV RNA detection percentages in bodies and saliva expectorants of Ae. aegypti IT inoculated with NHUV or NHUV and ZIKV by NHUV-specific qRT-PCR. d NHUV RNA copy number of mosquito bodies and salivary expectorants