Figures & data
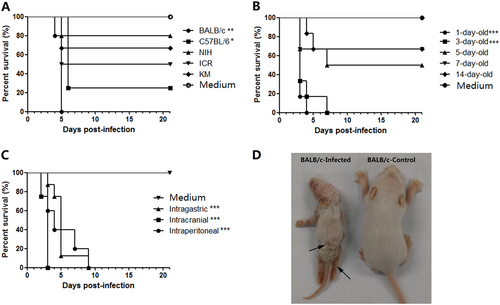
a The process of selecting a suitable mouse strain. One-day-old mice of various strains (BALB/c, C57BL/6, KM, ICR, and NIH) were i.p. challenged with CV-B5/JS417 (3.16 × 107 CCID50/mouse). b The process of selecting the suitable age. BALB/c mice at 1, 3, 5, 7, and 14 days of age were i.p. challenged with CV-B5/JS417 (3.16 × 107 CCID50/mouse). c The process for selecting the suitable inoculation route. Three-day-old BALB/c mice were challenged with CV-B5/JS417 (3.16 × 107 CCID50/mouse) via the i.p., intracerebral (i.c.), or intragastric (i.g.) route. The control mice were treated with medium via the same corresponding routes. n = 6 to 10 mice for each group. All mice were monitored daily for body weight and clinical symptoms for 21 dpi. One representation of two independent experiments was shown. The Mantel–Cox log-rank test was used to compare the survival of the pups between each group and the medium control group at 21 days post-infection. ***p < 0.001. **p < 0.01.* p < 0.05 (d). A representative image of 3-day-old BALB/c mice infected with CV-B5/JS417 (the left side) or with medium (the right side) at 7 dpi is shown, and the infected mice exhibited the following clinical symptoms: wasting, hair loss, and hindlimb paralysis (arrows)
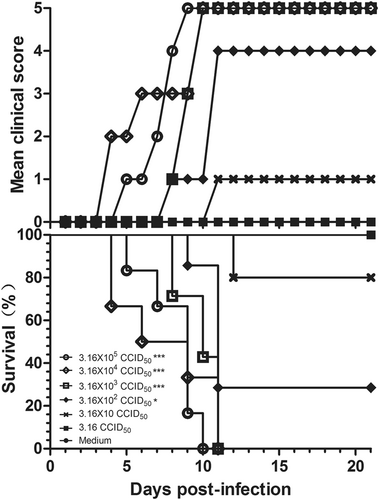
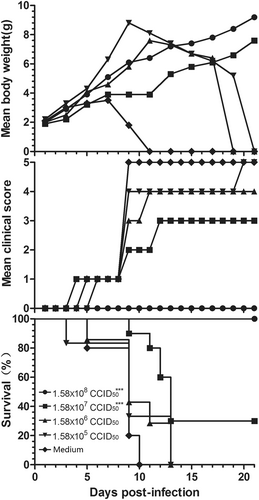
Three-day-old BALB/c mice (n = 6 to 10, per group) were i.p. inoculated with CV-B5/JS417 at a dose ranging from 3.16 to 3.16 × 105 CCID50/mouse (10-fold serially diluted). The control mice were treated with medium. The CV-B5/JS417-induced mean score of clinical symptoms and CV-B5/JS417-induced mortality were monitored and recorded daily. Representative results of duplicate experiments are shown. The Mantel–Cox log-rank test was used to compare the survival of the pups between each group and the medium control group at 21 days post-infection. ***p < 0.001. *p < 0.05
Reproducibility of the CV-B5-challenged neonatal mouse model
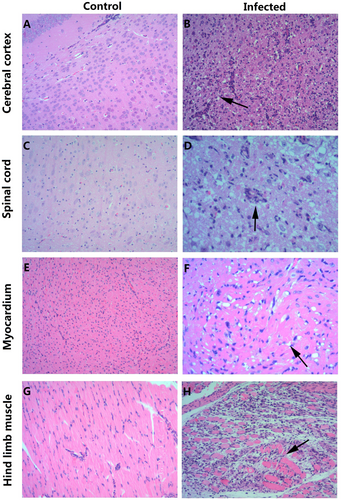
a, c, e, g HE staining of cerebral cortex, spinal cord, myocardium, and hindlimb muscle tissues of the control group, respectively. b, d, f, h HE staining of corresponding tissues of the experimental group. b Necrosis of the cerebral cortex associated with tubular infiltration (arrow); d: degeneration and necrosis of spinal cord nerve cells with glial response (arrow); f: eosinophilic necrosis of cardiomyocytes (arrow); h: necrotic myositis of hindlimb muscle (arrow). Magnifications ×100 (a–c, e, g, h), Magnifications ×200 (d, f). n = 6–10 mice for each group. One representative image is shown
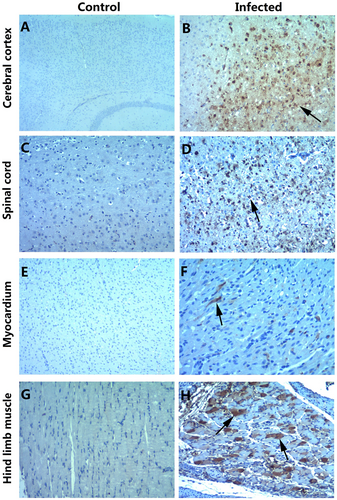
a, c, e, g IHC staining of the cerebral cortex, spinal cord, myocardium, and hindlimb muscle in the control group, respectively. b, d, f, h IHC staining of the cerebral cortex, spinal cord, myocardium, and hindlimb muscle in the experimental group. Positive staining was detected in the cerebral cortex (b, arrow), spinal cord (d, arrow), myocardium (f, arrow) and hindlimb muscle (h, arrow) of the neonatal mice after the intraperitoneal injection of CV-B5/JS417. Magnifications ×40 (a); magnifications ×100 (b–h). n = 6–10 mice per group. One representative image is shown
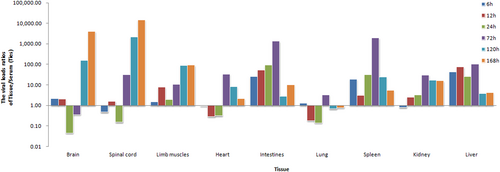
Three-day-old BALB/c mice were i.p. inoculated with CV-B5/JS417 (3.16 × 103 CCID50/mouse). To exclude the impact of the viral load in the serum, the viral load ratio (tissue/serum) was calculated by dividing the mean viral load of each tissue with the viral load of the serum at 6, 12, 24, 72, 120, and 168 h post-challenge (n = 3, per time point)
Adult female BALB/c mice were vaccinated with formaldehyde-inactivated CV-B5/JS417 (experiment group) or medium (control group) twice with a 2-week interval and allowed to mate 1 h after the first vaccination. The resulting pups were challenged with CV-B5/JS417 (3.16 × 103 CCID50/mouse) on day 3 after birth. The mortality, clinical symptoms, and body weight were monitored and recorded daily after the infection (n = 6 to 10, per group). One representative result is shown. The Mantel–Cox log-rank test was used to compare the survival of the pups between each vaccine group and the medium control group at 21 days post-infection. ***p < 0.001
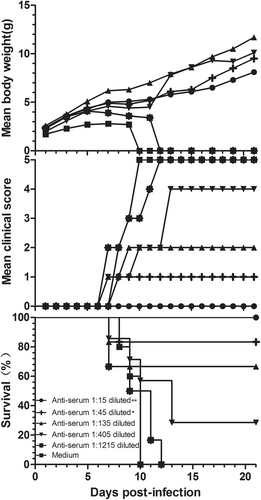
Serially diluted anti-CVB5 serum (neutralizing antibody 1:768) or medium was incubated with an equal volume of 3.16 × 103 CCID50 CV-B5/JS417 at 37 °C for 1 h. 3-day-old BALB/c mice (n = 6–10, per group) were i.p. inoculated with the diluted serum. The mortality, clinical symptoms, and body weight were monitored and recorded daily after the infection. One representative result of two independent experiments is shown. The Mantel–Cox log-rank test was used to compare the survival of the pups between each anti-serum group and the medium control group at 21 days post-infection. **p < 0.01, *p < 0.05