Figures & data
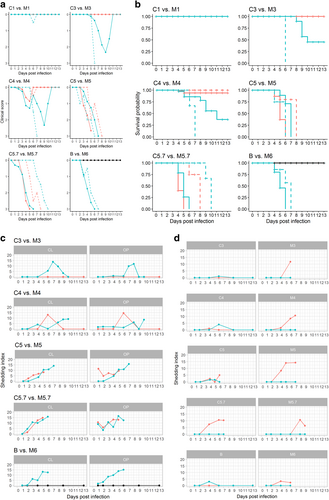
Fig. 1 a-b: Clinical scores (a) and survival probability (b) of chickens after co-infection with LP/HPAIV H7N7 (groups C1-C5.7, orange lines) compared with titer-matched HP H7N7 mono-infected groups (M1-M5.7, turquoise lines), and comparison between titer-matched LP (B, black line) and HP H7N7 (M6, turquoise line) mono-infected groups. Continuous lines represent inoculated animals; dashed lines depict sentinels. c–d: Oropharyngeal (OP) and cloacal (L) shedding of HPAIV H7N7-specific RNA by infected (c), and sentinel (d) chickens after co-infection with LP/HPAIV H7N7 (groups C1-C5.7, orange lines) compared with titer-matched HP H7N7 mono-infected groups (M1-M5.7, turquoise lines), and comparison between titer-matched LP (B, black line) and HP H7N7 (M6, turquoise line) mono-infected groups. A pathotype-specific RT-qPCR was used to generate Cq values (Graaf, 2017). The shedding index (Y-axes) was calculated by computing the mean average of Cq values of all animals sampled at the indicated dpi in a specific group and subtracting this value from 38, the threshold of detection of the HP H7 RT-qPCR. Thus, if all animals were negative for HP H7 RNA, the group scored with a value of zero
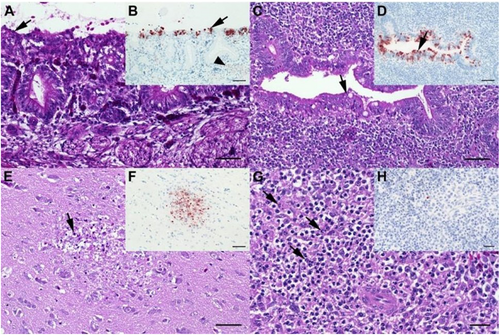
a Chicken, P17–882, group C5.7, respiratory mucosa. Moderate, multifocal, acute, necrotizing rhinitis with epithelial degeneration characterized by attenuation, loss of cilia (arrow), and sloughing. b Chicken P17–882, group C5.7, respiratory mucosa with coalescing foci of influenza A matrixprotein-immunoreactive (“antigen-positive”) morphologically intact and degenerated epithelial cells (arrow). Notably, there are scant immunoreactive granules in some of the submucosal nerves (arrowhead), suggestive of axonal spread. c Chicken, P17–903, group M5.7, cecum. Mild, oligofocal, acute, crypt epithelial degeneration with nuclear pyknosis (arrow), necrosis and sloughing. d Chicken, P17–903, group M5.7, cecum. Antigen-positive morphologically intact and degenerated crypt epithelia (arrow). e Chicken, P17–903, group M5.7, brain. Mild, oligofocal, acute, necrotizing polioencephalitis characterized by neuroglial cytoplasmic hypereosinophilia, nuclear pyknosis, karyorrhexis and loss (arrow), as well as an associated status spongiosus interpreted as inflammatory edema. f Chicken, P17–903, group M5.7, brain. Antigen-positive neuroglial cells within necrotizing lesion. g Chicken, P17–903, group M5.7, spleen. The periarteriolar lymphoid sheaths and follicles display moderate, coalescing apoptotic lymphocytes characterized by cytoplasmic hypereosinophilic shrinkage and nuclear karyorrhexis (arrows). h Chicken, P17–903, group M5.7, spleen. Rare, individual antigen-positive round cells can be detected in the white and red pulp. a, c, e, g: Hematoxylin eosin. b, d, f, h: Influenza A virus-matrixproteinIHC. a–f: bar = 50 µm. g, h: bar = 20 µm
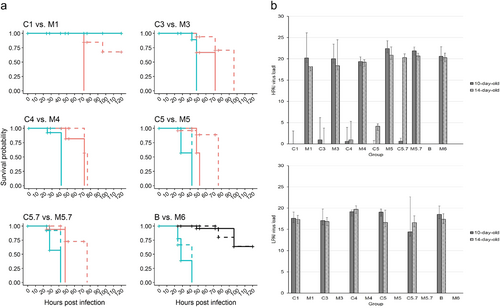
a. Survival probability of embryonated chicken eggs (ECEs) inoculated at 10 or 14 days of incubation with LP (group B, black lines in lower right panel “B vs. M6”) or HPAIV H7N7 (groups M1-M6, turquoise lines) or with LP/HP mixtures according to Table (groups C1-C5.7, orange lines). Solid lines represent ECEs 10-day old and dashed lines 14-day old at inoculation. b. HP (upper panel) and LPAI (lower panel) H7N7 virus loads in amnio-allantoic fluids of ECEs aged 10-and 14-days at inoculation determined by H7 pathotype-specific RT-qPCRs (mean values representing “virus load” were calculated by substracting the measured Cq value from the threshold of detection at Cq = 38; individual values are presented in supplemental Table 3a)
a Chicken, 14-day old embryo, P18–103, group B, 4 dpi, chorioallantoic membrane. There is necrosis and loss of the allantoic membrane and accumulation of cellular debris (arrow) and fibrin in the allantoic sac. b Chicken, 14-day old embryo, P18–103, group B, 4dpi, chorioallantoic membrane. Influenza A matrixprotein-immunoreactive (“antigen-positive”) cells and cellular debris accumulations restricted to the allantoic side of the chorioallantoic membrane. c Chicken, 12-day old embryo, P18–46, group C5.7, 4dpi, chorioallantoic membrane. Moderate, multifocal, acute, necrotizing vasculitis (arrow) with edema and extravascular accumulation of erythrocytes (arrowhead) interpreted as hemorrhage. d Chicken, 12-day old embryo, P18–46, group C5.7, 4dpi, chorioallantoic membrane. Influenza A-immunoreactivity is abundant in vascular endothelia (arrow) and blood-borne leukocytes and to a lesser degree epithelial cells (arrowheads) of both the chorionand the allantois (bottom). a, c: Hematoxylin eosin. b, d: Influenza A virus-matrixprotein IHC. a–d: bar = 20 µm
EID50/0.5 mL per chicken used for the in vivo experiment
EID50/0.2 mL per egg used for the in ovo experiment
