Figures & data
Figure 1. Magnetic resonance imaging and quantitative assessment of gadolinium in endolymphatic relative perilymphatic space. Sagittal section of the mouse brain showing the inner ear and the contrast enhancement in the cochlea (left). To evaluate the effect of spironolactone on EH in different contexts, the size of the endolymphatic fluid compartment, the relative area of scala media in the basal turn of the cochlea, was estimated by calculating the ratio between scala media (noncontrast enhanced, lower dotted area) and scala media + scala vestibule (scala vestibule, upper dotted area, is contrast enhanced) (right). The ratio of areas is subsequently converted to percentage as shown in . SV: scala vestibule; ST: scala tympani; SM: scala media; 1st: first turn; 2nd: second turn.
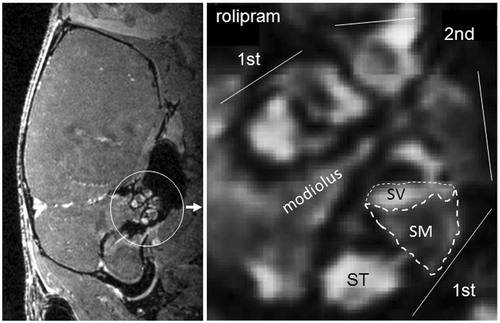
Figure 2. Spironolactone prevents vasopressin-induced endolymphatic hydrops in CBA/J mice. CBA/J mice were treated chronically with vasopressin for 28 d with or without SL and thereafter analyzed using MRI. Quantification of endolymphatic and perilymphatic cochlear compartments before and after vasopressin (VP) and before and after vasopressin plus spironolactone (VP + SL) administration was performed as described in Material and methods and in . Data are presented as means (left), as individual values for each ear before and after treatment (middle) and as individual values for each ear with prevalues set to 1 (right).
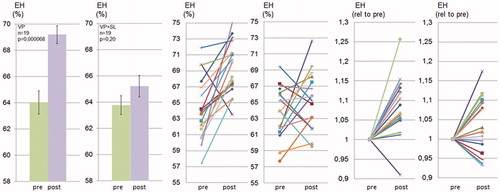
Figure 3. Spironolactone prevents rolipram-induced endolymphatic hydrops in CBA/J mice. CBA/J mice were treated chronically with rolipram with or without SL for 28 d and thereafter analyzed using MRI. for 28 d. Quantification of endolymphatic and perilymphatic cochlear compartments before and after rolipram (rol) and before and after rolipram plus spironolactone (rol + SL) administration was performed as described in Material and methods and in . Data are presented as means (left), as individual values for each ear before and after treatment (middle) and as individual values for each ear with prevalues set to 1 (right).
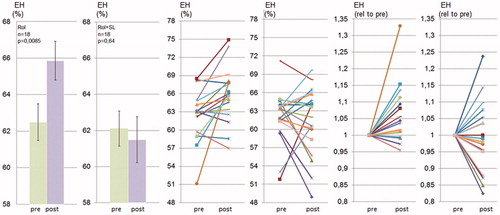
Figure 4. Spironolactone does not prevent cilostamide-induced endolymphatic hydrops in CBA/J mice. CBA/J mice were treated chronically with cilostamide with or without SL for 28 d and thereafter analyzed using MRI. Quantification of endolymphatic and perilymphatic cochlear compartments before and after cilostamide (Cil) and before and after cilostamide plus spironolactone (Cil + SL) administration was performed as described in Material and methods and in . Data are presented as means (left), as individual values for each ear before and after treatment (middle) and as individual values for each ear with prevalues set to 1 (right).
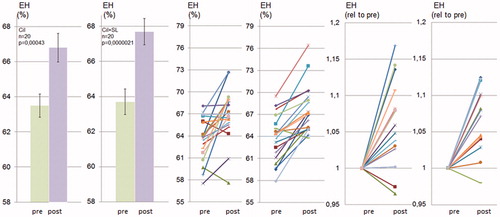
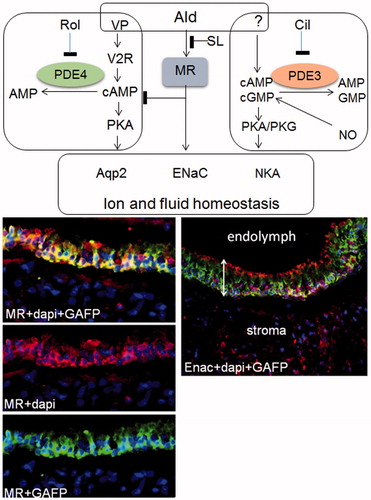
Figure 5. Pathway-dependent effect of spironolactone on the prevention of endolymphatic hydrops-working hypothesis. SL prevents EH induced by vasopressin believed to act via cAMP/protein kinas A mediated phosphorylation and translocation of the fluid transporter aqp2 to regulate inner ear functions. It is possible that PDE4 regulates the pool of cAMP utilized by vasopressin since (a) PDE4 interacts with aqp2 in kidney cells (b) PDE4 is expressed in the inner ear and (c) SL prevents hydrops induced by the PDE4 inhibitor rolipram. On the other hand, SL does not prevent hydrops induced by the PDE3 inhibitor cilostamide indicating that different pools of cyclic nucleotides are involved in hydrops induction in this model, cAMPPDE4, cAMPPDE3 and cGMPPDE3. Inhibition of aldosterone action could interfere with the vasopressin/PDE4 signaling systems at different levels. Since SL prevents rolipram-induced hydrops, one level would be downstream of cAMPPDE4 production. Mechanisms mediating cilostamide induced EH remain to be elucidated, however, most likely involve NO/cGMP as well as cAMP down-stream targets. Target proteins for the signaling systems described, of relevance for EH, presumably involve fluid and ion transporters such as aqp2, ENaC, NCC and NaKATPase. A key target for aldosterone action, ENaC and the mineralocorticoid receptor were expressed in the human saccule. Tissue sections were immunohistochemically stained with indicated antibodies. Merged photos of MR, DAPI (nuclear label) and GAFP (upper, left), merged photo of MR and DAPI (middle, left), merged photo of MR and GAFP (lower, left), merged photo with ENaC, DAPI and GAFP (right). NKA: NaKATPase; MR: mineralocorticid receptor; GAFP: glial acidic fibrillary protein; dapi stains nuclei; sensory epithelium, bar with two arrow heads.