Figures & data
Figure 1 Structure of PbS-PVC membrane electrode: 1, sensitivity membrane; 2, PVC tube; 3, Ag/AgCl coated wire internal reference electrode; 4, internal solution; 5, electrode base; and 6, electrode wire.
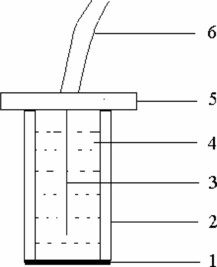
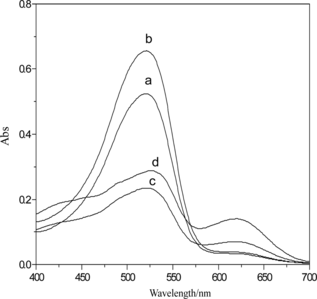
Figure 3 UV-vis spectrum of PbS nanoparticles: 1, H2Dz/CCl4; 2, Pb2+–H2Dz/CCl4; 3, PbS nanoparticles.
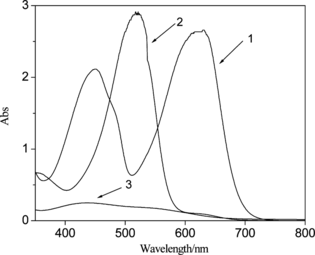
Figure 4 Variation of UV-vis spectrum with different acidity of Pb2+ ion solution: a = 4; b = 5; c = 6; d = 7; e = 8; f = 9; and g = 10.
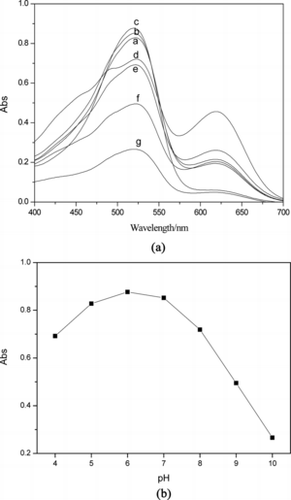
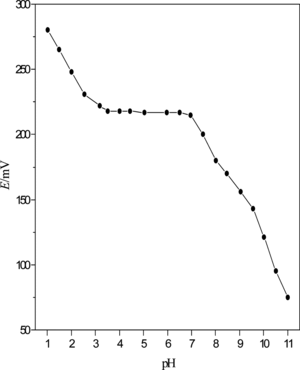
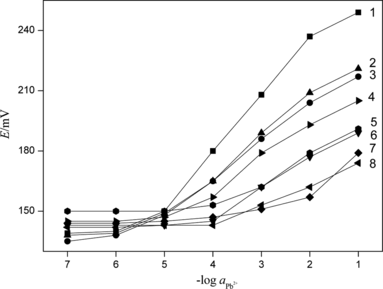
Figure 6 Influence of the potential response of Pb2+ selective electrode with different internal filling solution: a, 1 × 10−2 mol · L−1; b, 5 × 10−2 mol · L−1; and c, 1 × 10−1 mol · L−1.
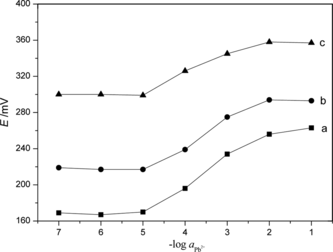
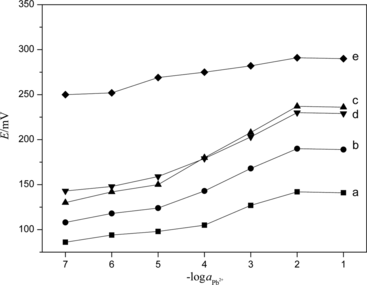
Figure 7 Variation of the potential of PVC membrane with different volume of PbS nanoparticles: a, 10 mL; b, 20 mL; c, 30 mL; d, 40 mL; and e, 50 mL.