Figures & data
Figure 1. Cytotoxic activity of three concentrations of silver nanoparticles (μg ml–1) from different weight ratios of silver metal (1, 3 and 5%) against MCF-7 cell line (survival %).
**Statistically significant at p ≤ 0.01.
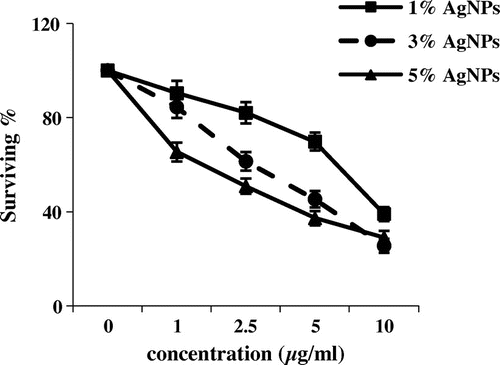
Figure 2. Cytotoxic activity of three concentrations of silver nanoparticles (μg ml–1) from different weight ratios of silver metal (1, 3 and 5%) against HepG2 cell line (survival %).
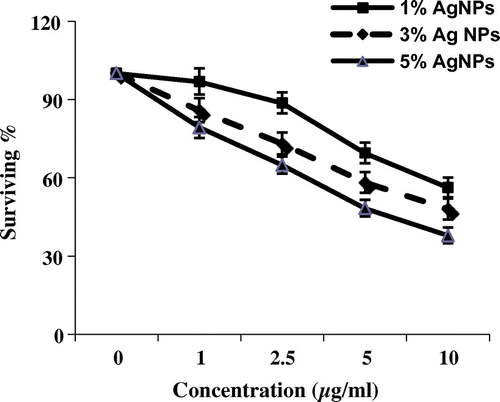
Figure 3. Mitotic index of Allium cepa meristems treated with silver nanoparticles. aStatistically significant at p ≤ 0.01.
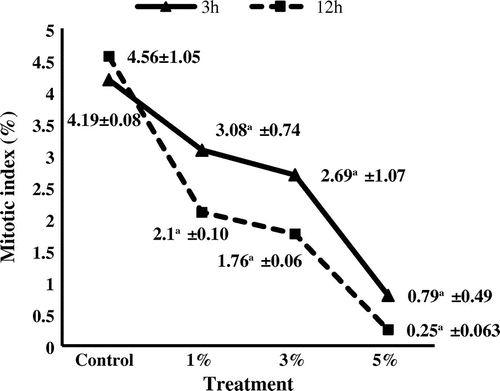
Figure 4. Types of chromosomal aberrations induced in Allium cepa meristems treated with silver nanoparticles. (a, b) irregular prophase; (c, d) c-metaphase; (e, f) disturbed anaphase; (g) sticky anaphase; (h) chromatin bridge; (i) chromosomal break and micronucleus in metaphase; (j) micronuclei in interphase; (k) uniucleate cell with two micronuclei (binucleate); (l) multinucleate cell in interphase.
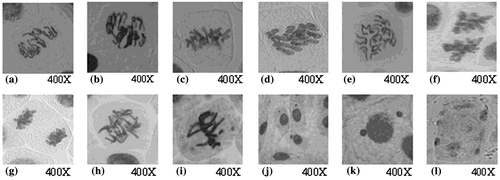
Figure 5. Percentage of micronucleus induced in Allium cepa meristems treated with silver nanoparticles.
aStatistically significant at p ≤ 0.05.
bStatistically significant at p ≤ 0.01.
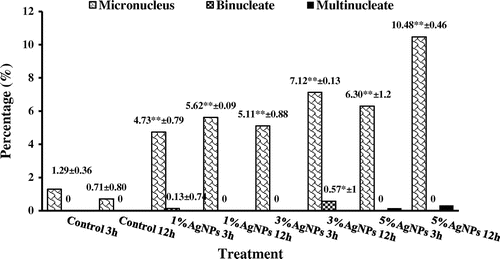
Figure 6. Change in protein profile of (a) Staphylococcus aureus and (b) Pseudomonas aeruginosa after treatment with silver nanoparticles.
(a) M: Marker; Sc: S. aureus control; S1%: S. aureus treated with 1% AgNPs; S3%: S. aureus treated with 3% AgNPs; S5%: S. aureus treated with 5% AgNPs.
(b) M: Marker; Pc: P. aeruginosa control; P1%: P. aeruginosa treated with 1% AgNPs;P3%: P. aeruginosa treated with 3% AgNPs; P5%: P. aeruginosa treated with 5% AgNPs.
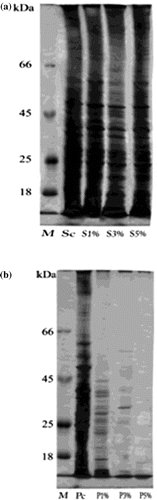
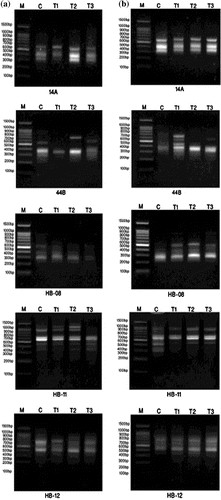
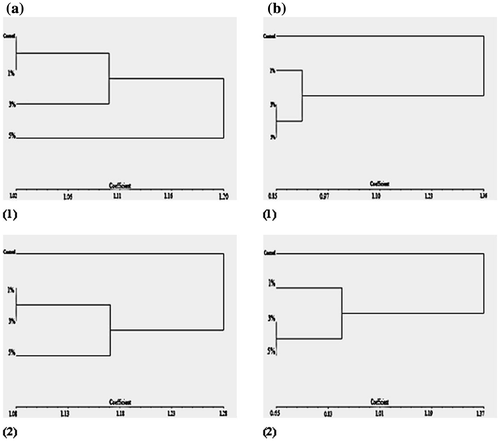
Figure 7. DNA polymorphism based on RAPD-PCR analysis of (a) Staphylococcus aureus and (b) Pseudomonas aeruginosa treated with silver nanoparticles.
(a) M: Marker; c: S. aureus control; T1: S. aureus treated with 1% AgNPs; T2: S. aureus treated with 3% AgNPs; T3: S. aureus treated with 5% AgNPs.
(b) M: Marker; c: P. aeruginosa control; T1: P. aeruginosa treated with 1% AgNPs; T2: P. aeruginosa treated with 3% AgNPs; T3: P. aeruginosa treated with 5% AgNPs.
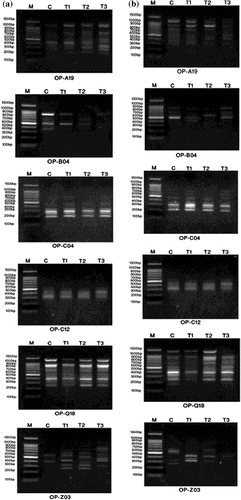
Figure 8. DNA polymorphism based on ISSR-PCR analysis of (a) Staphylococcus aureus and (b) Pseudomonas aeruginosa treated with silver nanoparticles.
(a) M: Marker; c: S. aureus control; T1: S. aureus treated with 1% AgNPs; T2: S. aureus treated with 3% AgNPs; T3: S. aureus treated with 5% AgNPs
(b) M: Marker; c: P. aeruginosa control; T1: P. aeruginosa treated with 1% AgNPs; T2: P. aeruginosa treated with 3% AgNPs; T3: P. aeruginosa treated with 5% AgNPs.