Figures & data
Figures 1-15. Longitudinal sections showing embryo sac development under bright field and fluorescence microscopes and different magnifications. 1: Young ovary containing a young ovule (right) showing dyad stage (right and left) (black arrowhead). 2: Two different magnifications of a T-shaped tetrad (black arrowheads). The outer integument (oi) grows faster than inner one (ii). 3: Three nuclei of four-nucleated embryo sac (black arrowheads). 4: Anatropous, crassinucellar and bitegmic ovules with nucellus and provascular bundle (PVB). The cone-shaped embryo sac with egg apparatus, two polar nuclei, antipodal cells and zigzag micropyle. 5: One of the synergids (SY) and oospher with its polarity are visible under bright field (black arrowheads) and fluorescence microscopes. 6: Synergids (black arrowheads), egg cell (EC), two polar nuclei before fusion and degeneration of micropylar end is visible. 7: Two polar nuclei and their finger-like tubers. 8: Egg apparatus and secondary nucleus (black arrowhead) formation after fusion of polar nuclei under bright field (left) and fluorescence (right) microscopes. 9: Mature embryo sac with differentiated egg (white arrowhead) and its polarity. 10: Sagittal section through the embryo sac under bright field and fluorescence microscopes with two different magnifications. Nucellar cells are degraded in the middle of embryo sac but persistent adjacent to the chalazal areae. Endothelium layer (En) is distinguishable at the inner integument. 11: Longitudinal section of zygote (Z) and synergids. 12: The primary endosperm nucleus (PEN), apical and basal cells (black arrowheads). 13: Basal cell and 2-celled proembryo (black arrowheads). 14: The U-shaped embryo sac and proembryo (Pe) under bright field (right) and fluorescence microscopes (left). The ovary with trichomes (in different magnifications; black arrowheads), long style (black arrow). 15: Globular embryo (Em) with young suspensor (S) surrounded by coenocytic endosperm. Staining with lugol indicates accumulation of starch grains at the base of embryo (black arrow).
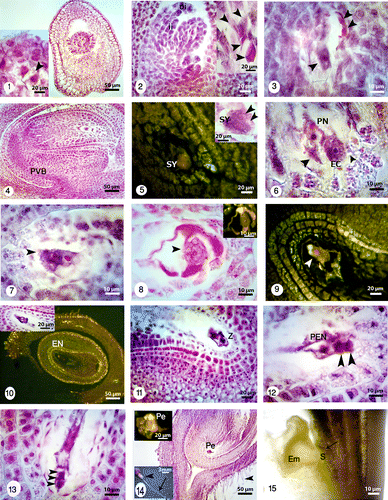
Figures 16-25. Different developmental stages of seed and embryo in Onobrychis persica under bright field and fluorescence microscopes with different magnifications. 16: Early heart-shaped embryo (EM) with suspensor (S) consisting of one column of cells. 17: The heart-shaped embryo (EM) and suspensor (S). 18: Onobrychis mature legume contained just one seed but the legumes with two seeds were also observed. 19: Longitudinal section of seed stained with methylene blue shows the distinguishable layers in seed. 20: Transverse section of seed stained with Sudan black B and polarizing microscopy shows lipid reserves in cuticle layer (C) and endosperm (EN) specially in aleurone layer (black arrowhead). 21: Staining of seed coat and endosperm with toluidine blue indicates starch grains (black arrowheads); positive reaction for phenolic compounds gives a green color. Starch grains are absent in macrosclereids (MS) but there are high accumulation of starch grains in osteosclereids (OS) and parenchyma cells (P). Cuticle layer (C) is thick. 22: Staining with lugol indicates starch grains (black arrows). Storage of starch grains during embryogenesis is visible in the seeds. The tracheid bar is distinguishable (white arrowhead). 23: At the hilar pole, double palisade layer (white arrowheads), subhilar parenchyma, and tracheid bar is distinguishable. The aleurone layer is also noticeable (black arrowhead) in the larger figure. 24: A part of testa showing macrosclereids (MS), osteosclereids (OS) and parenchyma cells (P), and accumulation of starch grains. 25: Cotyledon (CO) stage which accumulate starch. In this stage suspensor shows signs of degeneration (black arrow). Note: In Figures 19–25 seed sections were stained with different staining techniques and different microscopes were used (bright field, polarizing and fluorescence).
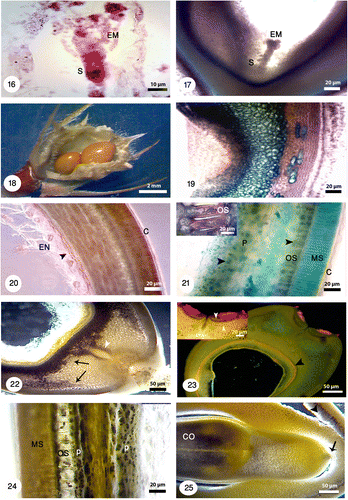
Figures 26-33. Anther structure under bright field and fluorescence microscopes with different magnifications. 26: Microspore mother cells surrounding by a callose coat (white arrow) in a young anther with undifferentiated wall (black arrow). The vascular bundles are distinguishable (white arrowhead). 27: Microspore mother cells are surrounded by a callose coat (black arrow). A big nucleus is visible at the center. 28: The arrangement of tetrads inside the callose walls is tetrahedral (white arrow) and tetragonal (white arrowhead). 29: Released microspores in polar and equatorial view (white arrowheads). The tapetal cells are uni-nucleate (white arrow). 30: Microspores undergoes mitotic division and produces a vegetative cell (black arrowhead) and a generative cell (black arrow). The anther wall consists of four layers: epidermis (E), endothecium (EN), the middle layer (ML) and a secretory tapetum (T). 31: Fibrous thickenings are developed in the endothecium (white arrowhead). 32: The anther is tetrasporangiate with thick exine schahuensis are T-shaped microspores (black arrow). The anther wall layers are distinguishable with fluorescence microscope (white arrow). 33: Two-celled mature pollen with generative and vegetative nuclei (black arrowheads) in a mature anther.
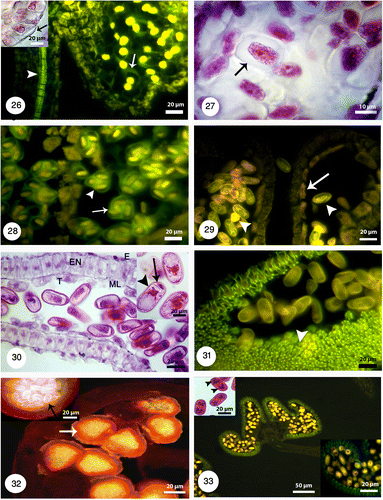
Figures 34-37. 34: Degeneration of micropylar end during fertilization (white arrow). Pollen tube growth with fluorescence microscopy and aniline blue staining: 35: Pollen tubes stained with aniline blue (white arrowheads) shows growing and branching on the tube in the space of between integuments. 36: Longitudinal section of the pistil showing pollen tube growth (white arrowheads) through the short open stylar canal that leads to an ovule. 37: Pollen tube growing through the micropyle (white arrowheads).

