Figures & data
Figure 1. Ki-67 immunostaining of the silver fox placenta at the 21st day of pregnancy. Glandular uterine epithelium (ge) shows high level of Ki-67 immunostaining whereas trabecular trophoblast (tr) shows a variety of Ki-67 immunostaining that depends, in particular, on the area and size of nuclei. Decrease of immunostaining is observed at the zone of cell detachment of trabeculae (arrows) and peaks in the zone of trophoblast cell destruction (double arrows), although some populations of intensively Ki-67-immunolabelled cells are found in this zone. At the bottom small nuclei of the developing labyrinth (lab) show high Ki-67 immunolabeling.
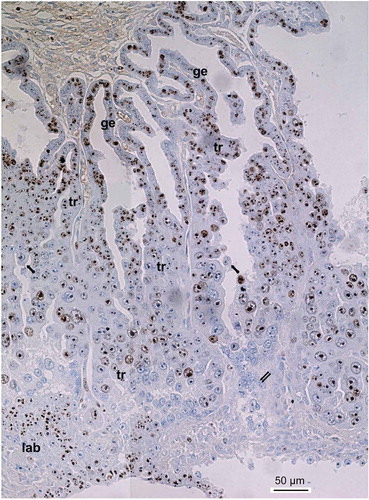
Figure 2. Immunolocalization of the Ki-67 protein in the trophoblast cells of the spongy zone of the silver fox at the 20th gd. (A) intensive Ki-67 immunostaining in the nuclei and karyoplasm of the low-ploid trophoblast cells close to the border with endometrium; (B–D) Ki-67 localization in the chromosome structures in the highly polyploid trophoblast cells; (C, D) Ki-67-positive chromatin strands (chromonemes) run from the nuclear envelope to nucleolus, as in Rabl orientation; the Ki-67-positive chromosome strands running in parallel into the nucleolus (arrowhead) prove some features of non-classic polytene chromosomes; (E–G) endomitotic (em) chromosomes show intensive Ki-67 immunostaining; (H–J) attenuation of Ki-67 immunolabeling in the region of trophoblast cell detachment from trabeculae: nucleoli longer retain the Ki-67 immunostaining, meanwhile endomitotic chromosomes often retain Ki-67 immunostaining longer than disappearing nucleoli; (K–N) subdivision of highly polyploid nucleoli into two or more ones via nuclear fragmentation (*); nucleoli and chromatin lose Ki-67 immunostaining. Magnification (Figure 2(k)) is the same for all photos.
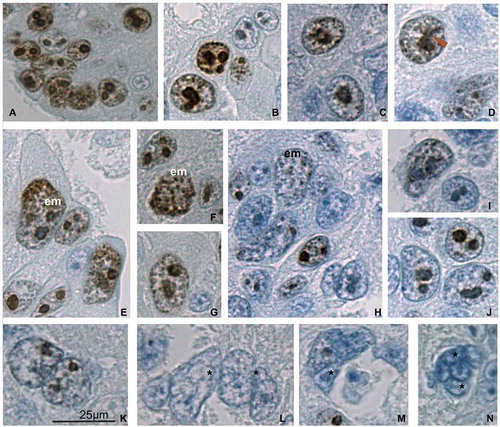
Figure 3. Ki-67 immunostaining of the trophoblast cells of spongy zone of silver fox at the 21st (A–K) and 22nd (L–N) gd. (A, B) Intensive Ki-67 immunostaining in the chromatin and nucleoli of the trabecular trophoblast cells close to the border with the endometrium (A) and in the middle of the spongy zone (B); (С) attenuation of Ki-67 immunostaining in the region detachment of the trophoblast cells from trabeculae; many nucleoli retain the dark immunostaining; (D, E, G, H) highly polyploid nuclei show Ki-67 immunopositivity in the non-classic polytene chromosomes (arrowhead); (D, E) heterogeneity of the trabecular trophoblast cell population: Ki-67-positive polytene nucleus, in endomitosis (em) attenuation of Ki-67 labeling with its relocalization onto endochromosomes, as well as nuclear fragmentation (*) of Ki-67 immunonegative nuclei and nuclei with residual labeling; (F) Ki-67 labeling of endochromosomes and nucleolus in endomitosis; (I–K) the trophoblast cells detaching from the trabeculae and undergoing cytoplasm destruction are mainly Ki-67 immunonegative, many nuclei undergo fragmentation (I, arrow); (L, M) at the 22nd gd many trabecular trophoblast cell nuclei undergo fragmentation (*), not infrequently they show Ki-67 immunopositivity in endomitosis (L, N).
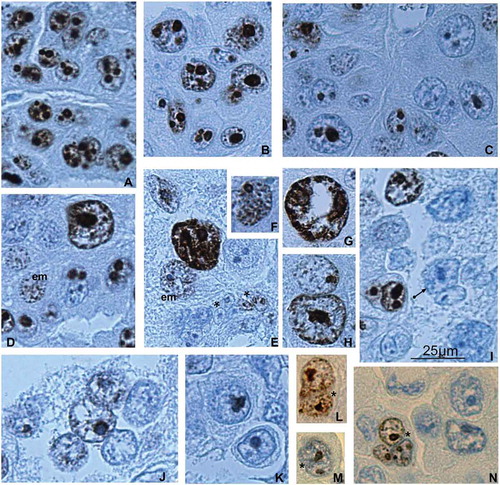
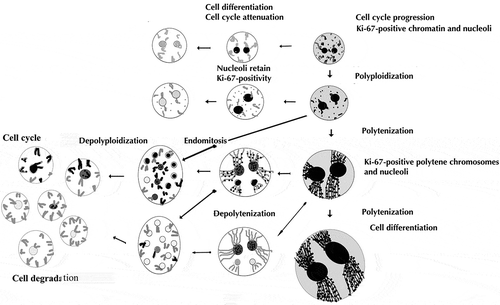
Figure 4. The hypothetical scheme of different modes of cell cycle modifications in the trabecular trophoblast cells in the course of their differentiation followed by degradation. In the region adjoining the endometrium the trophoblast cells actively proliferate and polyploidize via polyploidizing (reduced) mitoses, showing a high level of cell cycle progression; then some of the cells leave the cell cycle. The differentiated trabecular trophoblast cells continue genome reproduction cycles switching to endocycles – polytenization and endomitosis, thereby reaching high ploidy levels of 8–256c. The endomitotic nuclei may also result from depolytenization. In turn, it may lead to genome segregation. These processes are mostly accompanied by cell cycle attenuation with the exception of a minor cell population capable of cell cycle persistence. Ki-67-immunostained structures are shown by black color, Ki-67 immunonegative are gray.