Figures & data
Figure 1. A–E. The mature pollen grain of Tribulus terrestris: A, B. SEM; C–E. TEM. A. Whole, pantoporate grain; note pore (PO). B. The reticulum with polygonal lumina of varying size and shape. In each lumen there is a pore (PO); ectexinous granules (G) are seen on the aperture membrane (AM) and the muri (M) have pyramidal thickenings at the intersections of the muri (*). C. A section of a mature pollen grain [pre-fixed in glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4)] with a large central vacuole (V), numerous plastids (P), generative cell (GC); the sexine (SE) and nexine (NE) are interrupted by pores. D. Detail of the interporal region [material pre-fixed in glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4)]: sexine (SE), nexine (NE), ectexinous granules (G) on the pore membrane, murus (M), tryphine (T); intine (I), external layer of the intine (el), internal layer of the intine (il). E. Detail [material pre-fixed in glutaraldehyde then post-fixed in osmium tetroxide (OsO4)] of the mesopore (in Thiéry test) intine and tryphine show positive; the internal layer of the intine (il) is intensely positive, while the external layer (el) is moderately positive. Scale bars – 10 μm (A); 1 μm (B, E); 5 μm (C); 2 μm (D).
![Figure 1. A–E. The mature pollen grain of Tribulus terrestris: A, B. SEM; C–E. TEM. A. Whole, pantoporate grain; note pore (PO). B. The reticulum with polygonal lumina of varying size and shape. In each lumen there is a pore (PO); ectexinous granules (G) are seen on the aperture membrane (AM) and the muri (M) have pyramidal thickenings at the intersections of the muri (*). C. A section of a mature pollen grain [pre-fixed in glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4)] with a large central vacuole (V), numerous plastids (P), generative cell (GC); the sexine (SE) and nexine (NE) are interrupted by pores. D. Detail of the interporal region [material pre-fixed in glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4)]: sexine (SE), nexine (NE), ectexinous granules (G) on the pore membrane, murus (M), tryphine (T); intine (I), external layer of the intine (el), internal layer of the intine (il). E. Detail [material pre-fixed in glutaraldehyde then post-fixed in osmium tetroxide (OsO4)] of the mesopore (in Thiéry test) intine and tryphine show positive; the internal layer of the intine (il) is intensely positive, while the external layer (el) is moderately positive. Scale bars – 10 μm (A); 1 μm (B, E); 5 μm (C); 2 μm (D).](/cms/asset/dcbb5c8b-ae08-4c8b-8da6-71f9eef905e7/sgra_a_391796_o_f0001g.gif)
Figure 2. A–F. TEM of the Tribulus terrestris pollen tetrad stage. Early-mid tetrad stage, pre-fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with uranyl acetate and lead citrate. A. Part of a microspore, early tetrad stage: primexine matrix (PM) is fibrillar and thicker in the invaginate zones (*) than in the evaginate zones (thick arrows); note the plasmalemma (PL) and special callose wall (SCW). B. Detail of a microspore: pro-columellae (pC) initiated on the evagination; the primexine matrix increases in thickness (*) in the inter-columellar zones. C. Detail of a microspore: endoplasmic reticulum (ER, longer arrow) profile adjacent to the internal layer of the plasmalemma at a future/pro-aperture site (pA, short arrow). D. Microspore in the middle tetrad stage: the pro-murus (pM) is formed. E. Detail of (rectangle), the pro-columella (pC) and the pro-murus (pM) are heterogeneous with clear zones (arrow); the pro-columella is on the outer layer of the plasmalemma (PL). F. Aperture detail: the primexine matrix is micro-fibrillar, and comprises two layers: an outer layer (ePM), thick, with a loose mesh-like structure, which extends to the pore site (PO); and an inner layer (iPM) which fills the inter-columellar spaces but is absent at pore sites. Well-developed pro-columellae are apparent (pC) and, elements of the pro-muri (pM) of the future tectum can also be seen (arrow). Mitochondria (m), ribosomes (r) and endoplasmic reticulum (ER) are present in the cytoplasm. Scale bars – 0.5 μm (A); 0.2 μm (B, E, F); 2 μm (C, D).
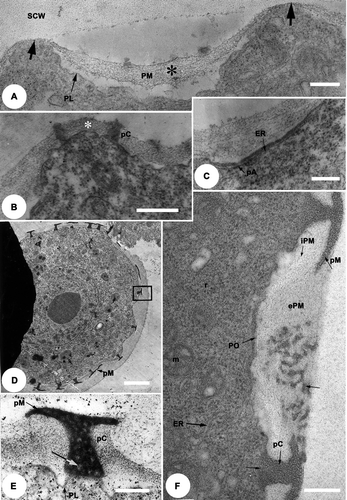
Figure 3. TEM of the late tetrad stage [A, B] to free microspore stage [C–E] of Tribulus terrestris pollen. A. Thiéry test: the outer layer of the primexine matrix (ePM) is moderately Thiéry positive while the inner layer (iPM) is strongly Thiéry positive. B. Fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) and stained with potassium permanganate (KMnO4). Callose digestion begins: primordial nexine lamellae (PNL) are initiated at the bases of the columellae; plasmalemma is present (PL), and the special callose wall (SCW) is still in place; cellular organelles are abundant. C. Part of a microspore wall fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) and stained with potassium permanganate (KMnO4); the columellae and the murus have thickened within the primexine matrix (PM), the primordial endexinous lamellae branch out (PNL & arrows), plasmalemma is still present (PL). D. General view of a microspore, fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) and stained with uranyl ucetate and lead citrate, showing development of endexinous lamellae. E. Detail of (box 1) at the mesoporal level: the wide spaces between the thin endexine lamellae (ENL) are filled with a fibrillar material (*), the plasmalemma (PL) is more pronounced. Sporopollenin deposition is initiated from the outside of the grain and continues towards the inner face of the endexine. The endexine is separated from the foot layer (FL) by a white line (paired arrows), the columellae (C) and muri (M) become thicker. Scale bars – 1 μm (A); 0.2 μm (B, C & E); 2 μm (D).
![Figure 3. TEM of the late tetrad stage [A, B] to free microspore stage [C–E] of Tribulus terrestris pollen. A. Thiéry test: the outer layer of the primexine matrix (ePM) is moderately Thiéry positive while the inner layer (iPM) is strongly Thiéry positive. B. Fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) and stained with potassium permanganate (KMnO4). Callose digestion begins: primordial nexine lamellae (PNL) are initiated at the bases of the columellae; plasmalemma is present (PL), and the special callose wall (SCW) is still in place; cellular organelles are abundant. C. Part of a microspore wall fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) and stained with potassium permanganate (KMnO4); the columellae and the murus have thickened within the primexine matrix (PM), the primordial endexinous lamellae branch out (PNL & arrows), plasmalemma is still present (PL). D. General view of a microspore, fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) and stained with uranyl ucetate and lead citrate, showing development of endexinous lamellae. E. Detail of Figure 3D (box 1) at the mesoporal level: the wide spaces between the thin endexine lamellae (ENL) are filled with a fibrillar material (*), the plasmalemma (PL) is more pronounced. Sporopollenin deposition is initiated from the outside of the grain and continues towards the inner face of the endexine. The endexine is separated from the foot layer (FL) by a white line (paired arrows), the columellae (C) and muri (M) become thicker. Scale bars – 1 μm (A); 0.2 μm (B, C & E); 2 μm (D).](/cms/asset/a56475e4-345a-4e4c-81a4-6effbd2ac99a/sgra_a_391796_o_f0003g.gif)
Figure 5. Schematic representation of exine development in Tribulus terrestris. Abbreviations: AM – aperture membrane, C – columella, EN – endexine, ENL – endexinous lamellae, FL – foot layer, G – ectexinous granules, L1 – ectexinous layer of the primordial nexine lamella, L2 – endexinous layer of the primordial nexine lamella, M – muri, NE – nexine, SE – sexine.
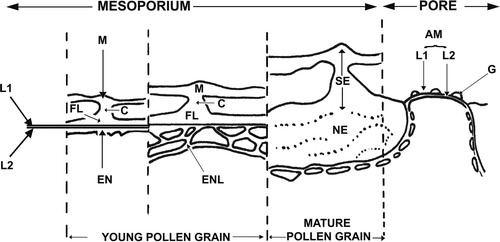
Figure 4. A–D. TEM of the development of the endexine: free microspore stage, in Tribulus terrestris pollen. A. Detail of (box 2): the pore (PO) is covered by an aperture membrane (AM) which is covered by ectexinous granules (G). Plasmalemma (PL, arrows) is continuous below the aperture membrane. B. The sporopollenin, fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4), deposition becomes more prominent on the endexine lamellae. C. The endexine [fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4)] lamellae thicken, and a more parallel alignment, together with a reduction in lumina size is apparent (arrows). D. The distal nexine, fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4), is now much more compact, and lamellae in the proximal/inner region are also notably compressed. Scale bars – 0.2 μm (A, B); 0.5 μm (C, D).
![Figure 4. A–D. TEM of the development of the endexine: free microspore stage, in Tribulus terrestris pollen. A. Detail of Figure 3D (box 2): the pore (PO) is covered by an aperture membrane (AM) which is covered by ectexinous granules (G). Plasmalemma (PL, arrows) is continuous below the aperture membrane. B. The sporopollenin, fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4), deposition becomes more prominent on the endexine lamellae. C. The endexine [fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4)] lamellae thicken, and a more parallel alignment, together with a reduction in lumina size is apparent (arrows). D. The distal nexine, fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4), is now much more compact, and lamellae in the proximal/inner region are also notably compressed. Scale bars – 0.2 μm (A, B); 0.5 μm (C, D).](/cms/asset/8c91732d-3b3f-4f6d-ae23-1169d4b27e1f/sgra_a_391796_o_f0004g.gif)