Figures & data
Table 1. Participant Demographics
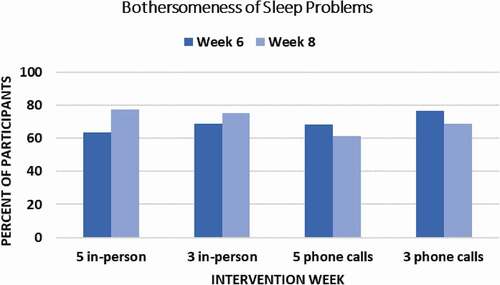
Figure 2. Program Evaluation and Treatment Satisfaction Ratings
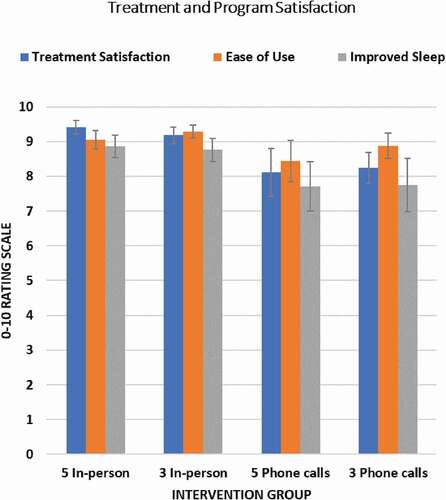
Figure 3. Percent of Participants in Each Group Achieving a Clinically Meaningful (≥ 0.5 SD) Improvement in Sleep Quality (PSQI) at Week 6 Endpoint and Week 8 Follow-Up
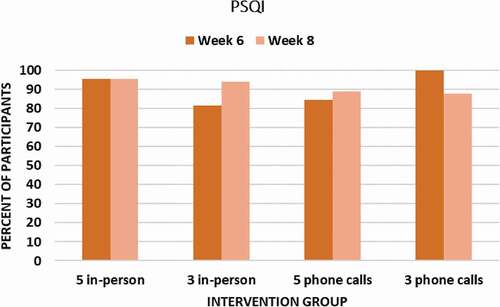
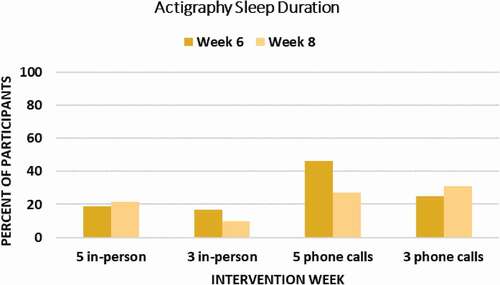
Figure 4. Percent of Participants in Each Group Achieving a Clinically Meaningful (≥ 0.5 SD) Improvement in Objective Sleep Duration (Wrist Actigraphy) at Week 6 Endpoint and Week 8 Follow-Up