Figures & data
Figure 1. Bee venom collector Beewhisper 5.0 is based on the electric stimulation protocol (http://www.beewhisper.com). In (A) it is shown how the equipment is put in front of the hive entrance. After completion of the collection cycle, the venom that has been deposited on the glass slide (B) can be scraped down.
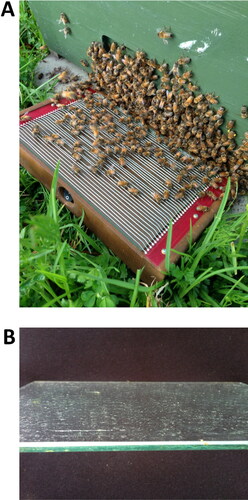
Figure 2. Manufactured device used to extract venom from individual honey bees; 1 = cork; 2 = piston; 3 = glass container; 4 = acrylic tube; smaller arrow = plastic; larger arrows = copper wire.
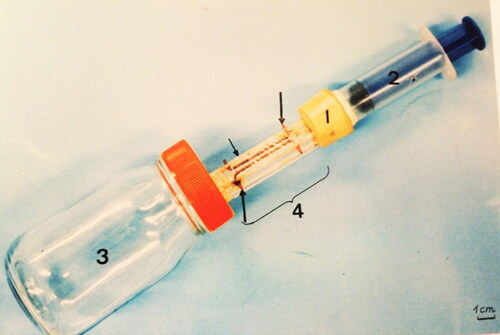
Figure 3. Steps of dissection and separation of the venom reservoirs from the sting apparatus. (A) Pull venom reservoir together with sting apparatus out of the bee's body using a fine-tipped tweezers; (B) with the two structures completely removed from the bee’s body, but still stuck together, use two tweezers (or one micro scissors and one tweezers) to separate one from another.
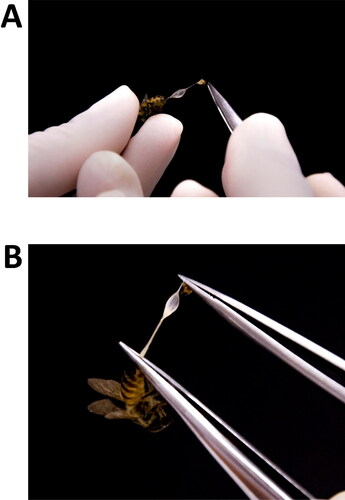
Table 1. ‘Pros and cons’ to help selecting the appropriate method for the determination of hyaluronidase activity.
Table 2. ‘Pros and cons’ to help selecting the appropriate method for the determination of PLA2 activity.
Table 3. ‘Pros and cons’ to help selecting the appropriate method for the determination of protease activity.
Figure 4. An example of a basophil activation test in a bee venom allergic patient. In panel A single cells are isolated from cell aggregates in a forward scatter plot FCS-H (height) versus FCS-A (area). In B, High IgE positive basophils are selected and analyzed in panel C for their positivity in CD203c (basophil specific). Cells stimulated with buffer (D) and anti-IgE (F) are analyzed as a negative and positive control, respectively. CD203c++ denotes activated basophils, CD203c++CD63+ denotes degranulating cells. In panels F-I cells stimulated with bee venom (F and G) or wasp venom (H and I) are analyzed. It shows clearly a positive dose dependent reaction to bee venom and not wasp venom as expressed by the higher proportion of CD203C++CD63+ degranulating cells.
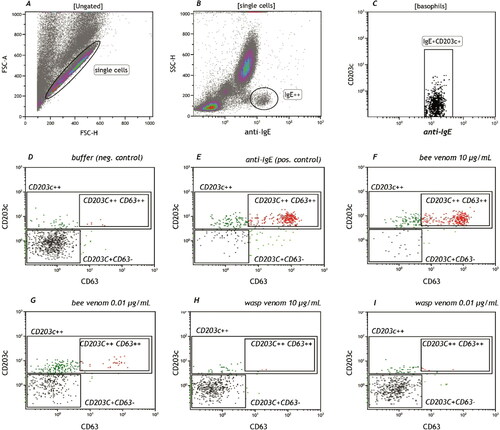
Figure 5. Basophils selected as in panels A-C in , were analyzed for their histamine content or DAO positivity. A and B are the necessary negative and positive controls. C-F is an example of a dose response curve in a bee venom allergic patient showing clearly that DAO positive cells (DAOpos) becoming CD63+ (x-axis) and start to release histamine (DAOrel). The dotted line is the difference between histamine positive and histamine releasing cells.
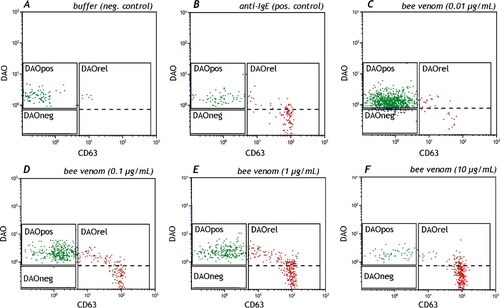
Figure 6. This figure shows the correct compensation setting as describe in . A is undercompensated, C is overcompensated and B shows the correct compensation setting.
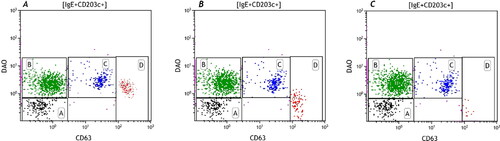
Table 4. Compensation method.
