Figures & data
Table 1. Purity of the respective R-BTP extractants and adsorption capacity of the R-BTP/SiO2-P adsorbents for Dy(III).
Table 2. Weight composition of the R-BTP/SiO2-P adsorbents obtained by TG-DTA.
Figure 2. Distribution coefficients (Kd ) of RE(III) on R-BTP/SiO2-P adsorbents in 3 M HNO3 solution (0.1 mM RE(III), phase ratio: 0.25 g/10 cm3, contact time: 72 h, temperature: 20°C).
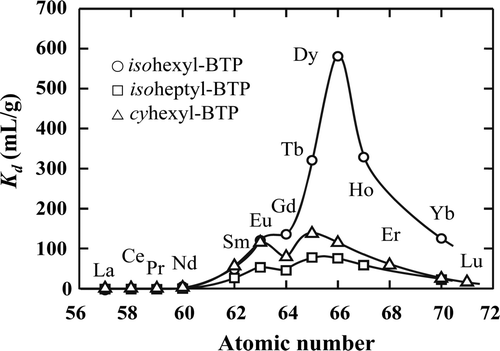
Table 3. Distribution coefficients (Kd ) of Am(III) and separation factors (SF) between Am(III) and Eu(III) on R-BTP/SiO2-P adsorbents in 3 M HNO3 at 25°C.
Table 4. Distribution coefficients (Kd ) of RE(III) on R-BTP/SiO2-P adsorbents in 3 M HNO3 solution.
Figure 3. Effects of contact time on desorption of several RE(III) ions loaded in isohexyl-BTP/SiO2-P adsorbent with H2O at 50°C (adsorption conditions: 1 mM RE(III), 3 M HNO3, 50°C, 5 h).
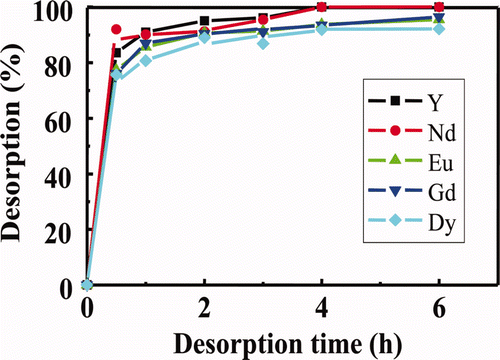
Figure 4. Effects of γ-ray irradiation on Kd of Dy(III) with isohexyl-BTP/SiO2-P adsorbent in 0.01 M HNO3 solution (dose rate: ≈40 Gy/h, room temperature).
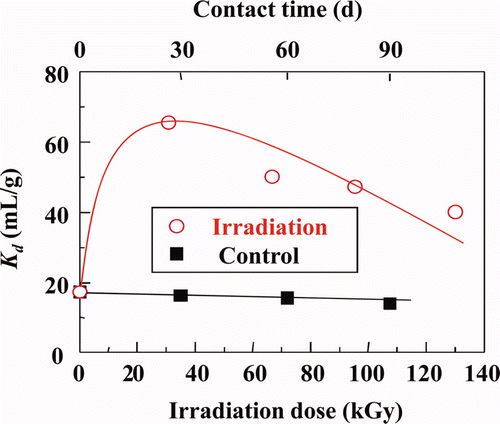
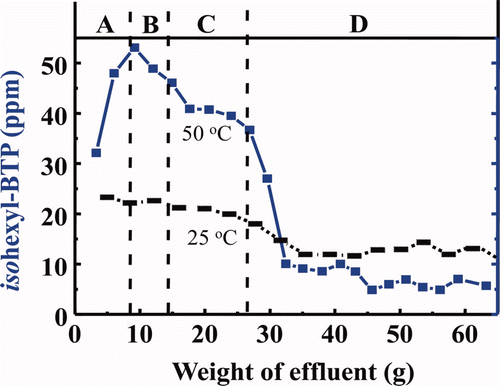
Figure 5. Effects of γ-ray irradiation on leakage of isohexyl-BTP extractant from isohexyl-BTP/SiO2-P adsorbent to 3M HNO3 solution (dose rate: ≈40 Gy/h, room temperature).
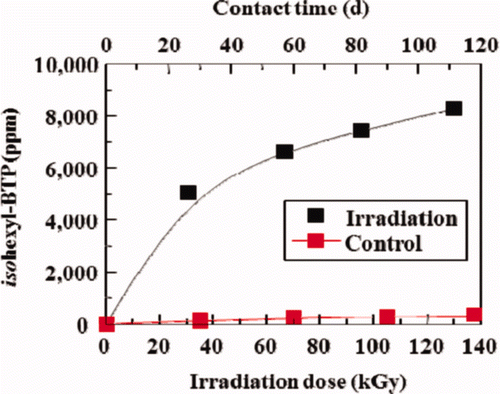
Table 5. Leakage of the R-BTP extractants from the R-BTP/SiO2-P adsorbents in contact with HNO3 solution.
Figure 6. Elution curves of various metal ions in simulated HLLW in a column experiment using isohexyl-BTP/SiO2-P adsorbent at 25°C (concentration of stable metal ions: 1 mM, 241Am and 99Tc: trace amounts, column size: φ8 mm × h150 mm, A: dead volume (3M HNO3), B: feed solution (3M HNO3), C: washing solution (3M HNO3), and D: eluting solution (H2O), flow rate: 0.25 cm3/min).
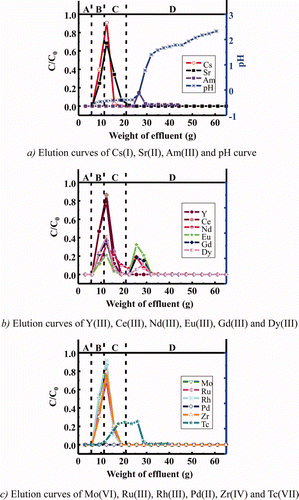
Figure 7. Elution curves of various metal ions in simulated HLLW in a column experiment using isohexyl-BTP/SiO2-P adsorbent at 50°C (concentration of stable metal ions: 1 mM, 241Am and 99Tc: trace amounts, column size: φ10 mm × h120 mm, A: dead volume (3M HNO3), B: feed solution (3M HNO3), C: washing solution (3M HNO3), and D: eluting solution (H2O), flow rate: 0.25 cm3/min).
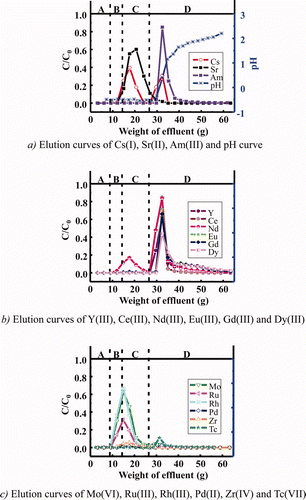
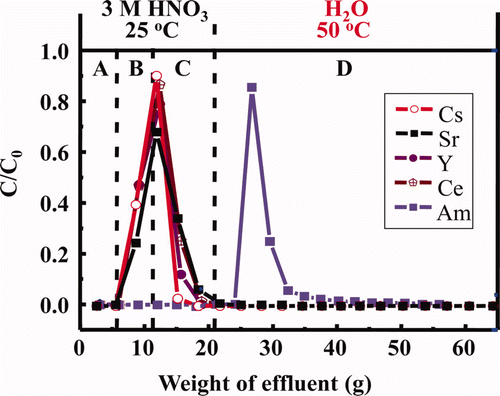
Figure 8. Estimated elution curves of Am and some FP ions using isohexyl-BTP/SiO2-P adsorbent under temperature control (A: dead volume, B: feed soln., C: washing soln., D: eluting soln., flow rate: 0.25 mL/min, C0 : initial concentration, C: concentration in effluent).