Figures & data
Figure 1. Activity of l-aminobutyric acid vs. mass of the material for the reaction. Gas sample material: HTO vapor; Reaction temp: 70°C; Reaction time: 1.0 h.
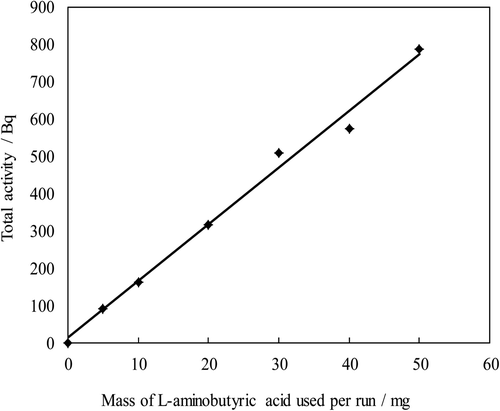
Figure 2. T specific activity vs. time for the reaction between l-aminobutyric acid and HTO vapor. Reaction temp: 25°C.
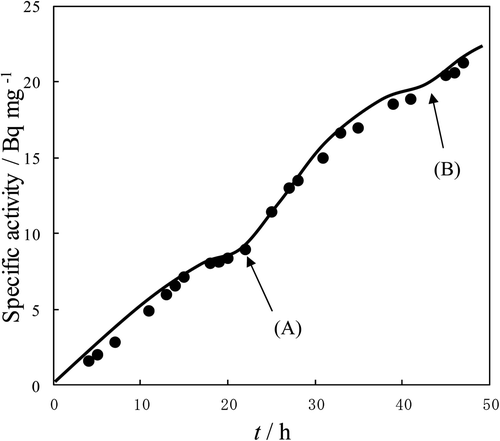
Figure 3. A″-McKay plots for l-aminobutyric acid in the reaction at 25°C. ▴: COOH group, •: NH2 group.
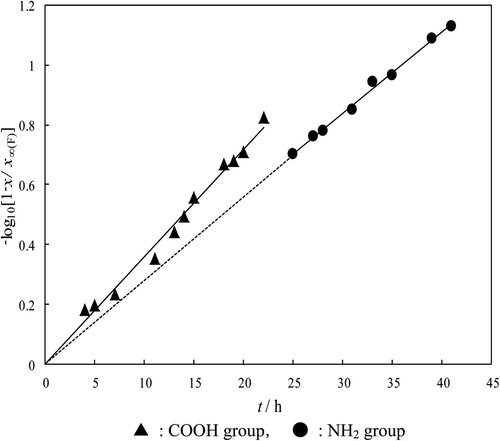
Table 1. Rate constants (k) for COOH and NH2 groups in each sample material and activation energy (E a).
Figure 4. Arrhenius plots for the COOH and NH2 groups in l-aminobutyric acid. ▵: COOH group, ○: NH2 group.
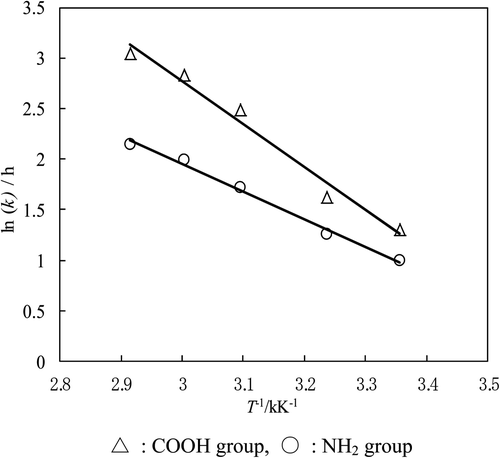
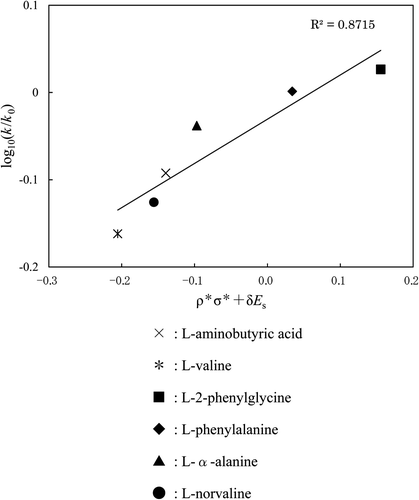
Figure 5. A plot (based on Taft's equation) of COOH group for each amino acid. Reaction temp: 25°C. ×: l-aminobutyric acid (obtained in this work), *: l-valine (obtained in this work), □: l-2-phenylglycine, ⋄: l-phenylalanine, ▵: l-α-alanine, ○: l-norvaline.
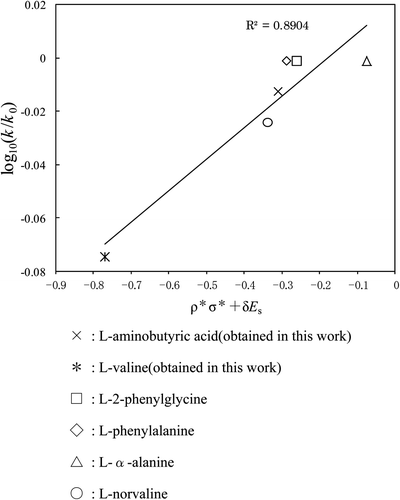
Figure 6. A plot (based on Taft's equation) of NH2 group for each amino acid. Reaction temp: 25°C. ×: l-aminobutyric acid, *: l-valine, ▪: l-2-phenylglycine, ♦: l-phenylalanine, ▴: l-α-alanine, •: l-norvaline.