Figures & data
Figure 1. An apparatus for denitration experiment. (a) A four necked separable flask, (b) a water bath, (c) a underwater magnetic stirrer, (d) a measuring cylinder, (e) a peristaltic pump, (f) a tygon tube, (g) a condenser tube, and (h) a recirculating cooler.
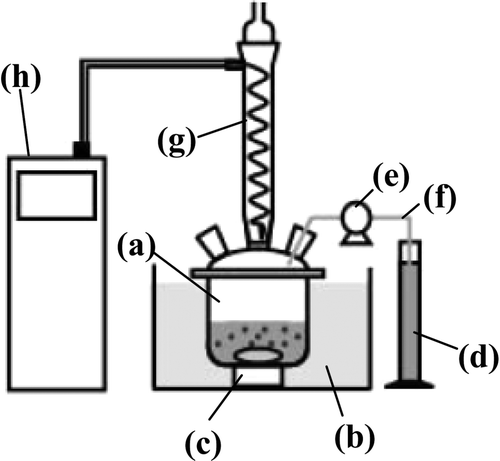
Table 1. Calculated and found ratios for the Pd and Cu supported on carbon powder.
Figure 2. XRD patterns for the carbon powders 0.47 Pd–x Cu/C (x = 0−0.39) and y Pd–0.39 Cu/C (y = 0−0.47). (a) Carbon powder, mole fraction of Cu/(Pd + Cu) = (b) 0, (c) 0.14, (d) 0.25, (e) 0.40, (f) 0.46, (g) 0.51, (h) 0.68, (i) 0.81, and (j) 1.
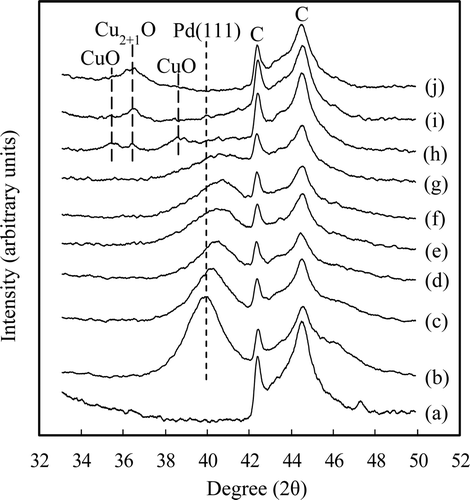
Figure 3. Concentrations of NO3 −, NO2 −, and N2H4 in suspension as a function of time. Reaction solution: 100 mL of 5 mol L−1 NaNO3, catalyst: 2 g of 0.47 Pd–0.31 Cu/C, reductant: 40 mL of N2H4 · H2O, drop rate of N2H4 · H2O: 80 mL h−1, temperature: 333 K. •: NO3 −, ○: NO2 −, □: N2H4.
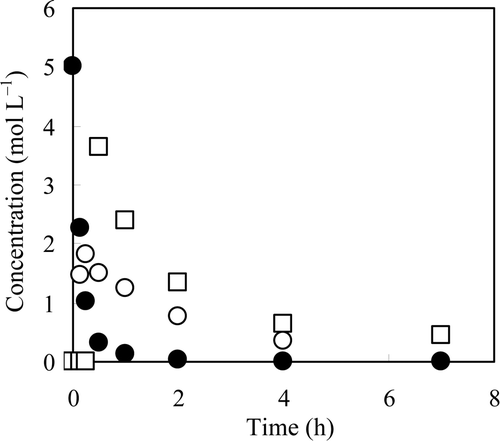
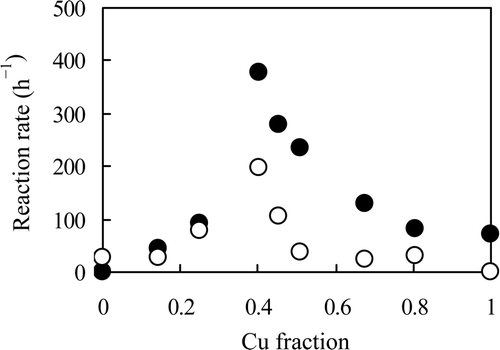
Figure 4. Reaction rates of NO3 − and NO2 − using several catalysts with different Cu fractions. Reaction solution: both 100 mL of 0.5 mol L−1 NaNO3 and NaNO2, catalyst: 0.2 g of 0.47 Pd–x Cu/C (x = 0−0.39) or y Pd–0.39 Cu/C (y = 0−0.47), reductant: 4 mL of N2H4 · H2O, temperature: 333 K. •: NO3 −, ○: NO2 −.