Figures & data
Table 1. Experimental variables and data for Se(IV) sorption on goethite.
Table 2. Experimental variables and data for Se(IV) sorption on ferrous oxide.
Table 3. Experimental variables and data for Se(IV) sorption on magnetite.
Table 4. Experimental variables and data for Se(IV) sorption on biotite.
Figure 1. Comparison of Kd values for Se(IV) obtained in this study (open marks) and previously reported ones (closed marks) for (a) goethite [Citation3,Citation12,Citation13,Citation15], (b) ferrous oxide, (c) magnetite [Citation21,Citation27], and (d) biotite [Citation3]. “0.01 M” represents the 0.01 mol dm−3 NaCl concentration. The previous data for ferrous oxide are not available.
![Figure 1. Comparison of Kd values for Se(IV) obtained in this study (open marks) and previously reported ones (closed marks) for (a) goethite [Citation3,Citation12,Citation13,Citation15], (b) ferrous oxide, (c) magnetite [Citation21,Citation27], and (d) biotite [Citation3]. “0.01 M” represents the 0.01 mol dm−3 NaCl concentration. The previous data for ferrous oxide are not available.](/cms/asset/35cb5fc7-31d5-41ee-a8c3-e07fec840572/tnst_a_864457_f0001_b.gif)
Figure 2. Experimental conditions of equilibrated solutions in a pH–Eh diagram for the H–O–Se system under standard conditions. The total concentration of Se is 10−8 mol dm−3. The circles, squares, triangles, and asterisks represent the conditions for the sorption experiments using goethite, ferrous oxide, magnetite, and biotite, respectively.
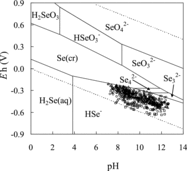
Table 5. Experimental variables and data for Se(−II) sorption on goethite (using 0.1 g of the solid phase).
Table 6. Experimental variables and data for Se(−II) sorption on goethite (using 1 g of the solid phase).
Table 7. Experimental variables and data for Se(−II) sorption on ferrous oxide.
Table 8. Experimental variables and data for Se(−II) sorption on magnetite.
Table 9. Experimental variables and data for Se(−II) sorption on biotite.
Figure 3. The Kd values for Se(−II) obtained in this study: (a) goethite, (b) ferrous oxide, (c) magnetite, and (d) biotite. Open marks and closed marks represent the data obtained using 1 g and 0.1 g of the solid phase, respectively.
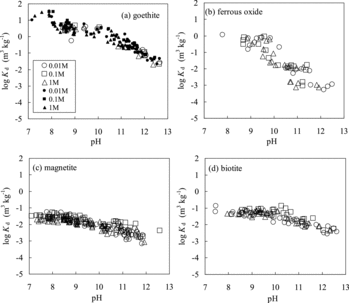
Figure 5. Comparison of Rs values of Se(IV) predicted using the TLM (lines) with experimentally measured ones (marks): (a) goethite, (b) ferrous oxide, (c) magnetite, (d) biotite (calculated with KintSeO3 = 1015.1), and (e) biotite (calculated with KintSeO3 = 1016.1).
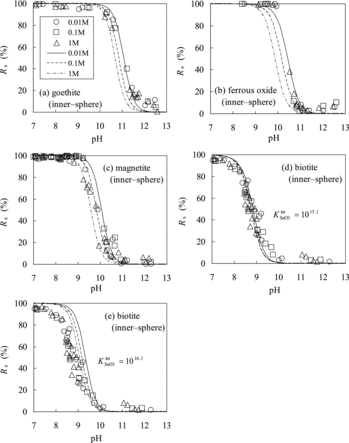
Figure 6. Comparison of Rs values of Se(−II) predicted using the TLM (lines) with experimentally measured ones (marks): (a) goethite (using 0.1 g of the solid phase; assuming inner-sphere complexation), (b) goethite (0.1 g of the solid phase; outer-sphere complexation), (c) goethite (1 g of the solid phase; inner-sphere complexation), (d) goethite (1 g of the solid phase; outer-sphere complexation), (e) ferrous oxide (inner-sphere complexation), and (f) ferrous oxide (outer-sphere complexation).
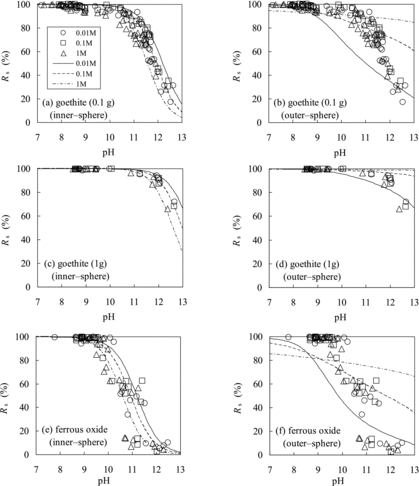
Figure 7. Comparison of Rs values of Se(−II) predicted using the TLM (lines) with experimentally measured ones (marks): (a) magnetite (assuming inner-sphere complexation), (b) magnetite (outer-sphere complexation), (c) biotite (inner-sphere complexation), and (d) biotite (outer-sphere complexation).
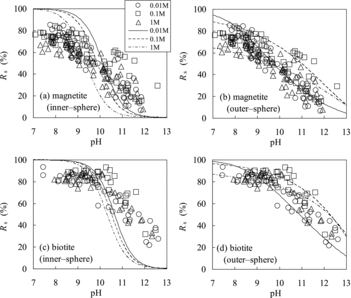
Table 10. Model parameters and equilibrium constants for the TLM calculation.