Figures & data
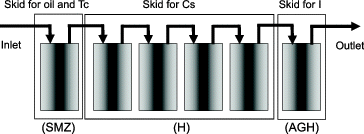
Figure 1. Diagram of water treatment system for highly contaminated water accumulated in Fukushima Daiichi NPS.
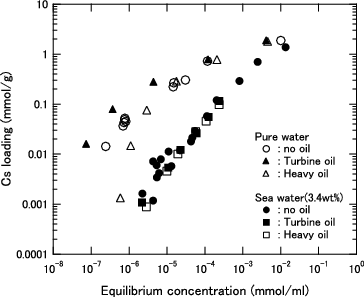
Figure 3. Cs loading as function of equilibrium Cs concentration for KURION herschelite in pure water.
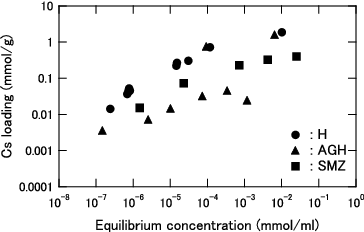
Figure 4. Cs loading as function of equilibrium Cs concentration for KURION herschelite in seawater (3.4 wt% salt).
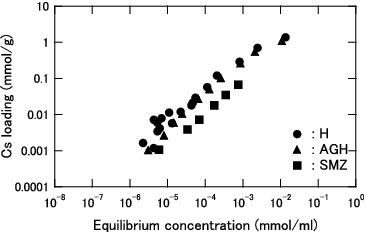
Figure 5. Distribution coefficient (Kd) for Cs as function of equilibrium Cs concentration for KURION herschelite in pure water.
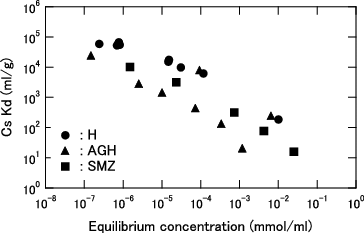
Figure 6. Distribution coefficient (Kd) for Cs as function of equilibrium Cs concentration for KURION herschelite in seawater (3.4 wt% salt).
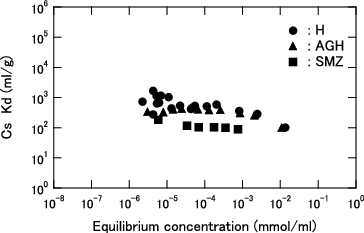
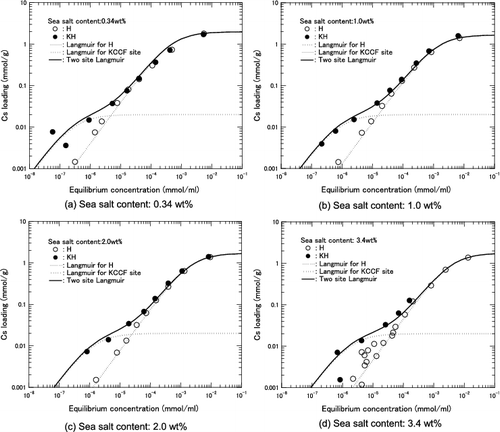
Figure 7. Cs loading as function of equilibrium Cs concentration for H herschelite in several sea salt containing solution. (Dashed lines: Results of calculation using Langmuir equation for each sea salt content).
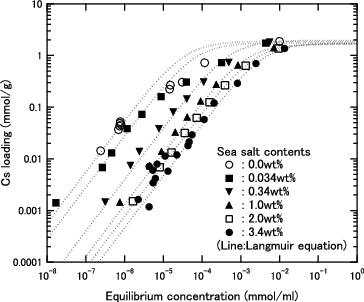
Figure 9. Cs loading as function of equilibrium Cs concentration for KH in several sea salt containing solutions.
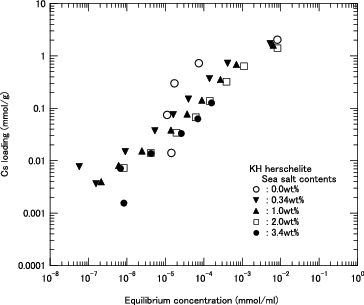
Table 1. Langmuir coefficients for H herschelite at various sea salt concentrations.
Table 2. Langmuir coefficients of KCCF site in two-site Langmuir equation for KH at each sea salt concentration.