Figures & data
Table 1. Radionuclides of concern for RDD production (as reported in [Citation11]).
Table 2. Samples tested in our studies, and the cladding materials investigated.
Table 3. Properties of the applied materials in the experiments.
Figure 1. Aerosol size distribution for the Co sample and its mixture with the cladding materials, obtained by weighing the MOUDI impactor plates before and after the experiments.
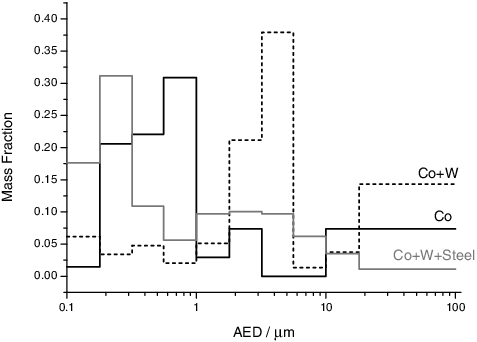
Figure 2. Examples of aerosols from the Co sample, showing the different types of particles: in the first stages (top left, with cut-off sizes 1.8 < AED < 18 μm), individual spherical particles were found; in stage 5 (top right, with a cut-off size of AED 1 μm), agglomerated particles were observed (a magnified image of these agglomerates is shown in the bottom-left frame). Nanometric agglomerates were found in the last stages 6–8 (bottom right, AED < 0.56 μm).
Figure 3. Raman spectrum measured for mixed cobalt–tungsten aerosols, showing all the typical bands of CoWO4, as reported in [Citation17–19].
![Figure 3. Raman spectrum measured for mixed cobalt–tungsten aerosols, showing all the typical bands of CoWO4, as reported in [Citation17–19].](/cms/asset/f7714631-fc9e-4581-a067-9fee38811973/tnst_a_1050473_f0003_b.gif)
Figure 4. Examples of aerosols from a mixed cobalt–tungsten sample (50/50%wt.), showing the different particles collected: top, aerosols collected from the first stages (1.8 < AED < 18 μm), isolated big particles and externally mixed agglomerates; bottom, mixed agglomerates containing Co and W, in the smaller particles, a higher content of tungsten was detected by SEM/EDX. The table shows the average concentration together with the maximum value detectable by EDX.
Figure 5. Aerosol size distribution for the CsCl sample and its mixture with the cladding materials, obtained by weighing the MOUDI impactor plates before and after the experiments. The size distribution refers to a CsCl + W + Steel mixture (25/50/25 composition), with may explain the minimal shift of the peak to μ = 1.11 μm (σ = 600 nm).
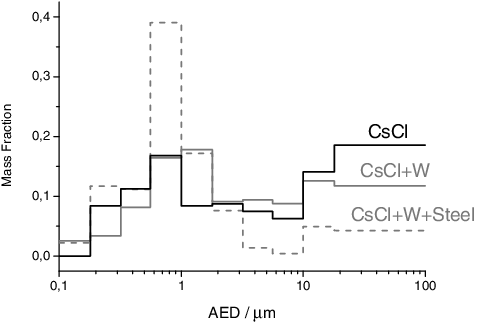
Figure 6. Results from the Raman analyses on the aerosols produced from a mixture of CsCl and W, showing a chemical partitioning over the different stages. Smaller particles show the presence of WO3. The middle stages show some modification in the spectra (e.g. 320, 700–780 and 900 cm−1 bands) that are related to the formation of a new chemical compound.
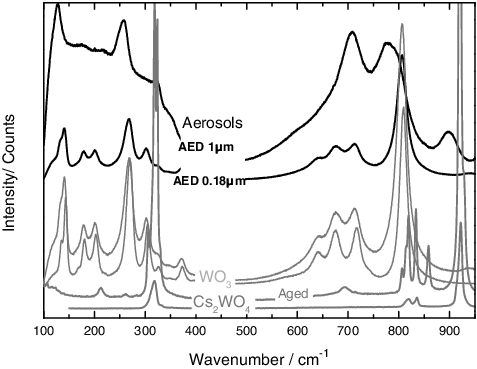
Figure 7. Aerosol size distribution for the SrTiO3 sample and its mixture with the cladding materials, obtained by weighing the MOUDI impactor plates before and after the experiments.
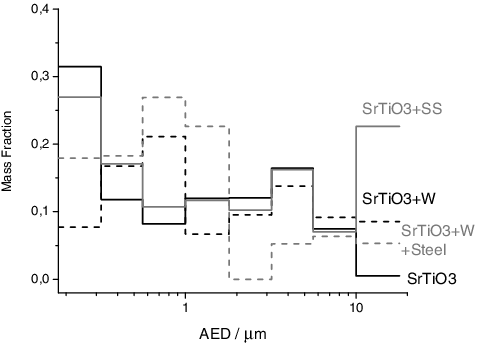
Figure 8. Raman spectra for the SrTiO3 aerosols and pellets, showing the difference between the bulk material and the first-order bands related to the nanometric aerosols. In particular, the spectrum of the particles in the stage 5 (AED of 1 μm) is similar to the bulk material, indicating that this effect becomes evident only for smaller particles.
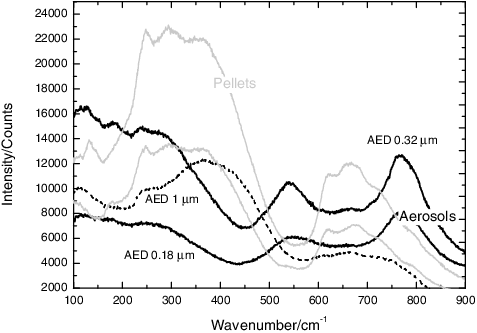
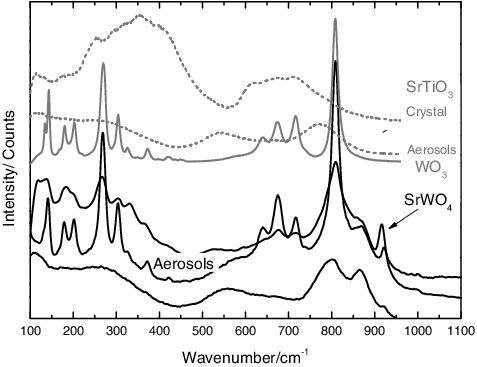
Figure 9. Typical Raman spectra for the aerosols collected from an SrTiO3 sample with tungsten, showing a chemical partitioning with AED. In the smaller particles, the bands of WO3 can be observed. The formation of SrWO4 can be inferred from the band at 922 cm−1.