Figures & data
Table 1. Chemical composition of the KMOC glass.
Table 2. Chemical compositions (wt%) of Pd metal phase and Ru oxide phase included in the KMOC glass. Numbers in parenthesis represent standard deviation.
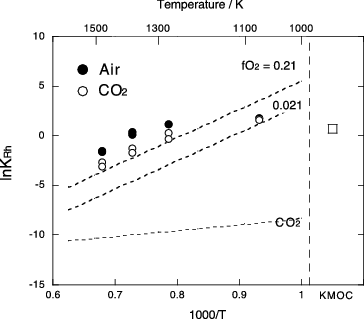
Figure 1. Temperature and oxygen fugacity of Pd/PdO, Rh/Rh2O3 and Ru/RuO2 equilibria calculated from FACT thermodynamic database [Citation30]. Dashed and dotted lines represent in air and equilibrium
of CO2 = CO+0.5O2, respectively. Experimental temperature and oxygen fugacity are indicated by solid circles.
![Figure 1. Temperature and oxygen fugacity of Pd/PdO, Rh/Rh2O3 and Ru/RuO2 equilibria calculated from FACT thermodynamic database [Citation30]. Dashed and dotted lines represent fO2 in air and equilibrium fO2 of CO2 = CO+0.5O2, respectively. Experimental temperature and oxygen fugacity are indicated by solid circles.](/cms/asset/47cb106d-9847-4cea-969b-e9731956946e/tnst_a_1050474_f0001_b.gif)
Figure 2. Methods of sample holding tested in this study. (a) Wire-loop method, (b) crucible method, (c) SiO2 tube method and (d) Al2O3 tube method. In all methods, molten samples are held by a surface tension between glass melt and Pt wire or tubes.
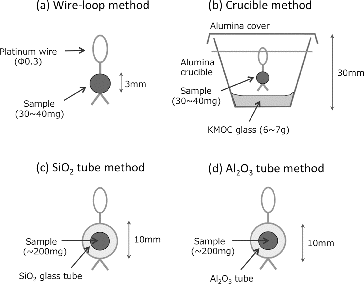
Figure 3. Plots of chemical compositions of the glass matrix after phase equilibrium experiments against temperature: (a) SiO2, (b) Al2O3, (c) B2O3 and (d) Na2O. Open squares with cross represent concentration in the starting KMOC glass.
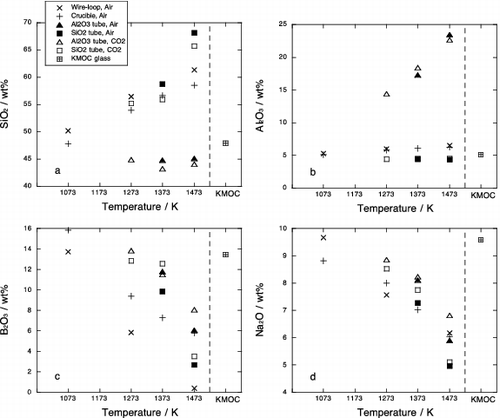
Table 3. Chemical compositions of the Pd metal phase and the Ru oxide phase crystalized in the phase equilibrium experiments and calculated partition coefficient of Rh (lnKRh). Values normalized to total 100wt%. Numbers in parenthesis represent standard deviation.
Table 4. Chemical compositions of the Pd metal phase and the platinum wire used for sample holding by the crucible method and the wire-loop method above 1373 K. Numbers in parenthesis represent standard deviation.
Figure 4. Chemical compositions of the Pd metal phase in experimental samples; (a) Pd, (b) Rh, (c) Te. Solid circle and open circle are results in air and in CO2 atmosphere, respectively. Open square represents composition of Pd metal included in the starting KMOC glass. Error bar is a standard deviation (1σ).
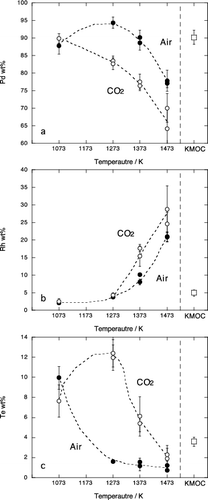
Figure 5. Chemical compositions of the Ru oxide phase in experimental samples; (a) RuO2 and (b) RhO2. Values normalized to total 100wt%. Solid circle and open circle are results in air and in CO2 atmosphere, respectively. Open square represents composition of Ru oxide included in the starting KMOC glass, respectively. Error bar is a standard deviation (1σ).
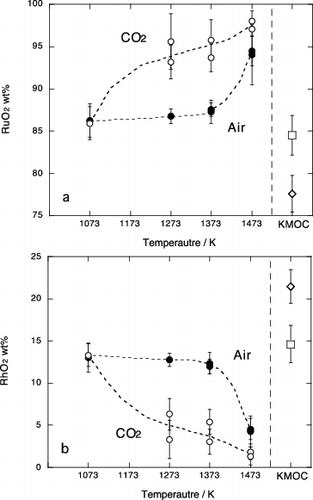
Figure 6. Mass fraction of the Pd metal phase and the Ru oxide phase in the sample of phase equilibrium experiments as a function of temperature. Amount in the KMOC glass is also shown.
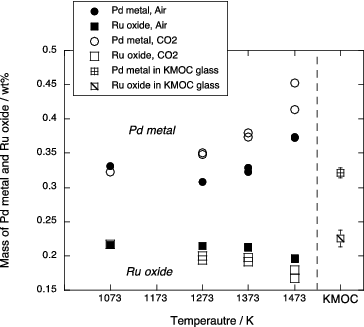
Figure 7. Partition coefficient of Rh (KRh) between Pd metal and Ru oxide. Solid circle and open circle are results in air and in CO2 atmosphere, respectively. Open square represents the KRh calculated from crystal compositions in the KMOC glass. Dash lines are the KRh calculated from thermodynamic data assuming three difference oxygen fugacities: 0.21(air), 0.021 and equilibrium of CO2 dissociation. Errors are comparable to symbol size.