Figures & data
Figure 1. Eh–pH diagram for Se based on the thermodynamic data provided by Kitamura et al. [Citation1]. The total concentrations of Se and Fe are 1 × 10−5 and 1 × 10−2 mol dm−3, respectively.
![Figure 1. Eh–pH diagram for Se based on the thermodynamic data provided by Kitamura et al. [Citation1]. The total concentrations of Se and Fe are 1 × 10−5 and 1 × 10−2 mol dm−3, respectively.](/cms/asset/d3c99131-6403-4951-b326-8c9be1ab1b7d/tnst_a_1137245_f0001_b.gif)
Figure 2. Eh–pH diagram for the prediction of Se aqueous species based on the thermodynamic data provided by Kitamura et al. [Citation1]. The total concentration of Se is 1 × 10−7 mol dm−3. The experimental data obtained from the over- and under-saturation directions are plotted as open circles and solid symbols, respectively.
![Figure 2. Eh–pH diagram for the prediction of Se aqueous species based on the thermodynamic data provided by Kitamura et al. [Citation1]. The total concentration of Se is 1 × 10−7 mol dm−3. The experimental data obtained from the over- and under-saturation directions are plotted as open circles and solid symbols, respectively.](/cms/asset/60235d0e-f129-404b-ab94-726122379509/tnst_a_1137245_f0002_b.gif)
Figure 3. Titration of 0.80 mol kg−1 NaCl with 0.0100 mol dm−3 HCl using a combination glass electrode. [H+free, add] is the moles of added free acid per liter and [H+obs] = 10−pHobs, where pHobs is the observed pH measured with a combination glass electrode. [H+obs] as a function of [H+free, add] is shown as open circles. The solid line denotes the linear least-squares fit of data. The logarithm of the slope of this line corresponds to B = 0.03. This value is required to convert the pHobs reading to the −log10 [H+] value of the samples used in this study, using the following equation: −log10 [H+] = pHobs + B.
![Figure 3. Titration of 0.80 mol kg−1 NaCl with 0.0100 mol dm−3 HCl using a combination glass electrode. [H+free, add] is the moles of added free acid per liter and [H+obs] = 10−pHobs, where pHobs is the observed pH measured with a combination glass electrode. [H+obs] as a function of [H+free, add] is shown as open circles. The solid line denotes the linear least-squares fit of data. The logarithm of the slope of this line corresponds to B = 0.03. This value is required to convert the pHobs reading to the −log10 [H+] value of the samples used in this study, using the following equation: −log10 [H+] = pHobs + B.](/cms/asset/c0221a3a-c612-431e-9b6d-3a2e3c5f9033/tnst_a_1137245_f0003_b.gif)
Figure 4. XRD pattern of the solid sample from 158-day aged suspension produced from the over-saturation direction. The pH of this suspension was 6.08.
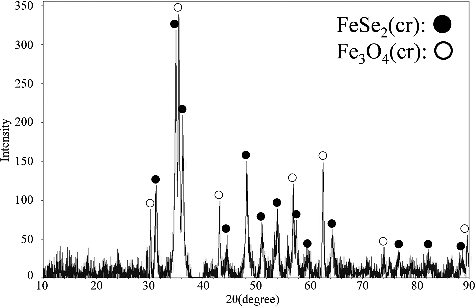
Figure 5. XRD pattern of the solid sample from 105-day aged suspension produced from the under-saturation direction. The pH of this suspension was 6.64.
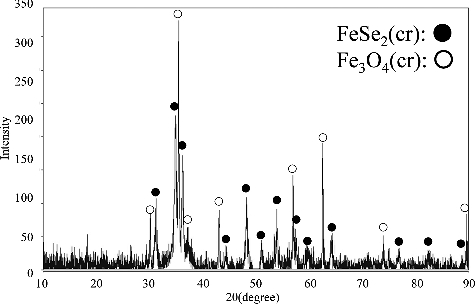
Table 1. Summary of solubility experiments of Se performed from both the over- and under-saturation directions in the presence of Fe under moderately reduced and neutral pH conditions.
Figure 6. Eh–pH diagram for Fe based on the thermodynamic data provided by Lemire et al. [Citation12]. The total concentration of Fe is 1×10−7 mol dm−3. The experimental data obtained from the over- and under-saturation directions are plotted as open circles and solid symbols, respectively.
![Figure 6. Eh–pH diagram for Fe based on the thermodynamic data provided by Lemire et al. [Citation12]. The total concentration of Fe is 1×10−7 mol dm−3. The experimental data obtained from the over- and under-saturation directions are plotted as open circles and solid symbols, respectively.](/cms/asset/33472333-e110-4eed-9350-da2c1d9fc8f4/tnst_a_1137245_f0006_b.gif)
Figure 7. Determination of the n value for FenSe which is the solubility-controlling solid and the log10K○5 value for FenSe dissolution reaction represented by (4FenSe(cr) ⇌ 4nFe2+ + Se42− + (8n − 2)e−) using the SIT model. The value of () is plotted against the value of (
). Solubility data obtained from the over- and under-saturation directions are shown as open circles and solid symbols, respectively. The solid line denotes the linear least-squares fit of data. The slope and intercept of this line correspond to −4n = −2.01 ± 0.09 and log10K○5 = −17.09 ± 0.28, respectively.
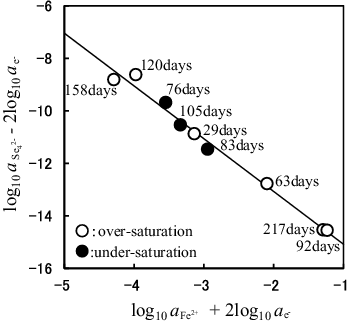
Figure 8. Eh–pH diagram for Fe based on the thermodynamic data provided by Lemire et al. [Citation12]. The total concentrations of Fe are 1 × 10−7 mol dm−3 (solid line) and 1 × 10−4 mol dm−3 (dotted line). The experimental data obtained from the over- and under-saturation directions are plotted as open circles and solid symbols, respectively.
![Figure 8. Eh–pH diagram for Fe based on the thermodynamic data provided by Lemire et al. [Citation12]. The total concentrations of Fe are 1 × 10−7 mol dm−3 (solid line) and 1 × 10−4 mol dm−3 (dotted line). The experimental data obtained from the over- and under-saturation directions are plotted as open circles and solid symbols, respectively.](/cms/asset/af3809eb-8270-403e-94a9-87e5a5e2405d/tnst_a_1137245_f0008_b.gif)
