Figures & data
Figure 1. (a) Schematic of rod-type ER geometry; (b) flat-plate-type ER geometry for numerical analysis; and (c) electrorefiner for validation.
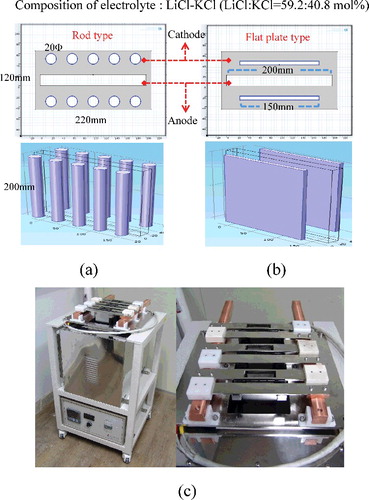
Table 1. Boundary condition and parameters.
Figure 2. (a) Grid of rod cathode using tetrahedral mesh; (b) grid of flat-plate cathode using mapped mesh; (c) changes in current density depending on changes in maximum mesh size.
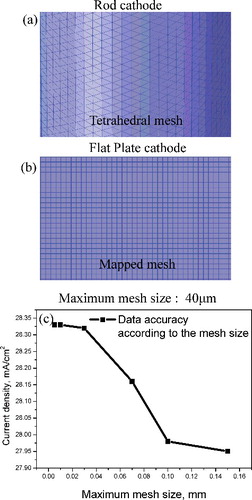
Figure 3. (a) Current density distribution on rod cathode and (b) flat-plate cathode. (c) Comparison of uniformity of current density distribution at locations on rod and flat-plate cathodes.
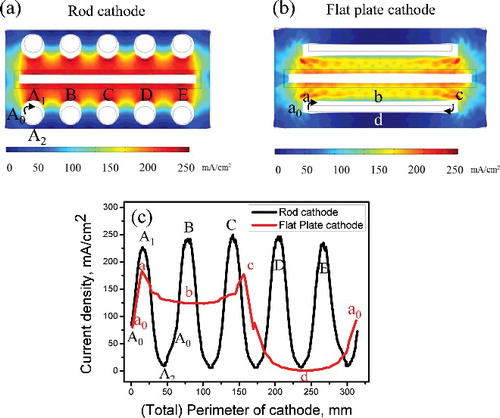
Figure 4. (a) Graph of anode dissolution on rod cathode and (b) flat-plate cathode. (c) Graph of cathode electrodeposition on rod cathode (d) flat-plate cathode.
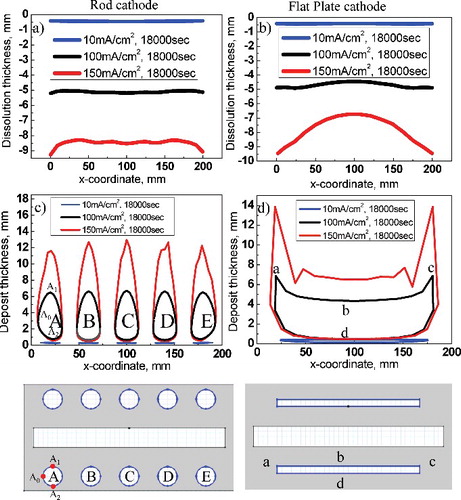
Figure 5. (a) Potential–current curve for rod and flat-plate cathode at No. 1 and No. 3. (b) Overpotential for rod and flat-plate cathodes as time passes at No. 1 and No. 3. (c) Uranium ion concentration depending for time spent at each location on rod and flat-plate cathodes.
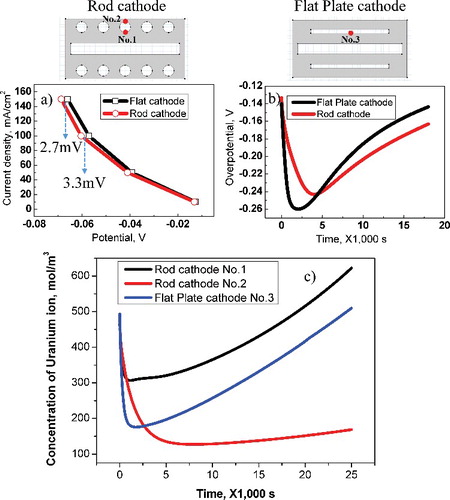
Figure 6. (a) Comparison of differences in overpotential with respect to rod cathode convection effect. (b) Comparison of differences of overpotential with respect to the flat-plate cathode convection effect.
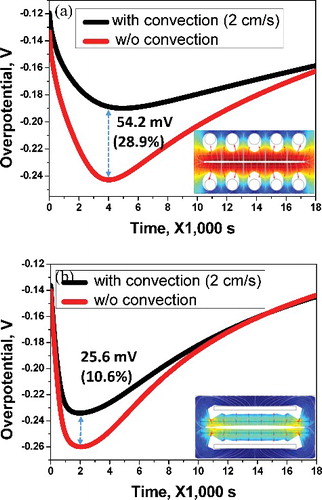
Figure 7. (a) Current density distribution on multi-arrayed (1 × 2, 3 × 4, 5 × 4) rod cathode; and (b) multi-arrayed (1 × 2, 3 × 4, 5 × 4) flat-plate cathode.
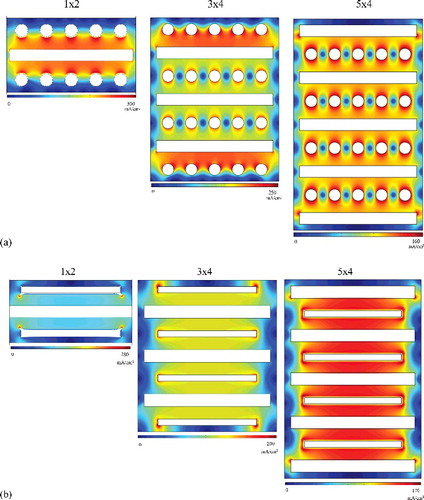
Figure 8. (a) Comparison of the differences in current density distribution on multi-arrayed (1 × 2, 3 × 4, 5 × 4) rod cathode, and (b) on multi-arrayed (1 × 2, 3 × 4, 5 × 4) flat-plate cathode.
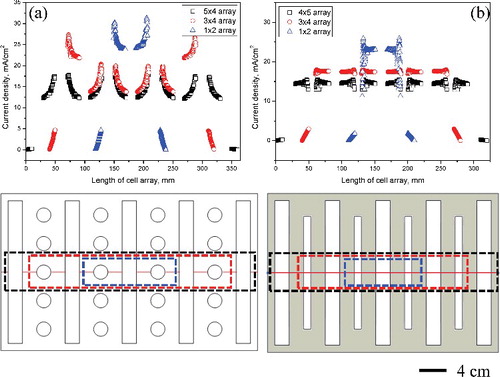
Figure 9. (a) Current density distribution, (b) overpotential curve according to surface area ratio, (c) calculated potential--current curve, and (d) experimental potential--current curve according to the surface area ratio of anode to cathode.
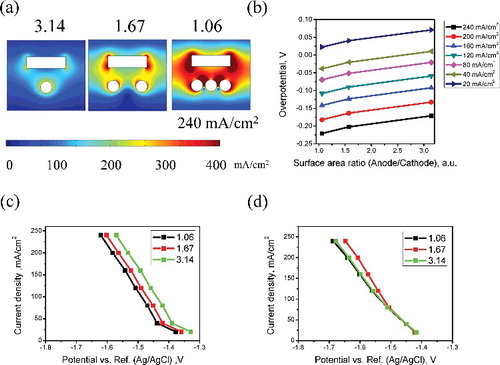
Table 2. Experimental current efficiency for U electrorefining with electrode surface area ratio.
