Figures & data
Table 1. Average size and atomic ratios of PtCu nanoparticles measured using TEM, ICP-AES, and XRD. The sample IDs note the pH of the reaction medium and the support used.
Figure 1. Metal ion ratio in solution as a function of pH of precursor containing (a) carbon support and (b) γ-Fe2O3 support (circle denotes Pt ion and square denotes Cu ion).
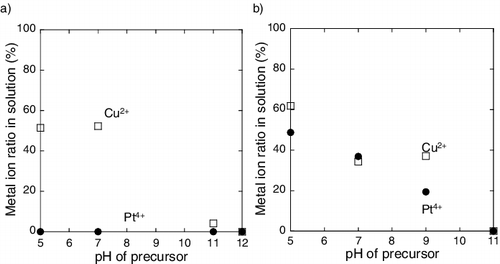
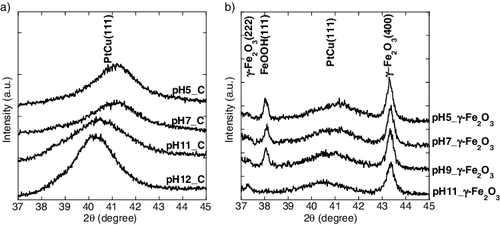
Figure 2. TEM micrographsaphs of PtCu nanoparticles supported on carbon at (a) pH 5, (b) pH 7, (c) pH 11, and (d) pH 12.
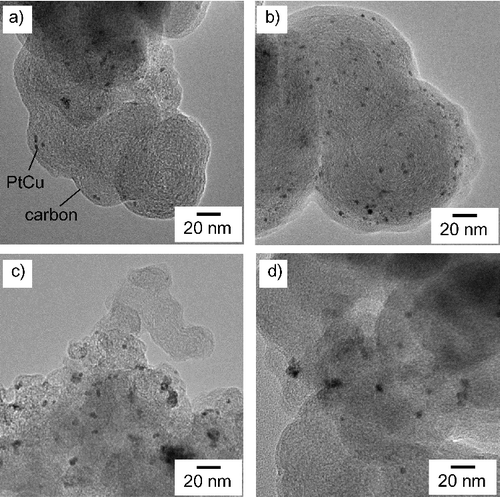
Figure 3. TEM micrographs of PtCu nanoparticles supported on γ-Fe2O3 at (a) pH 5, (b) pH 7, (c) pH 9, and (d) pH 11.
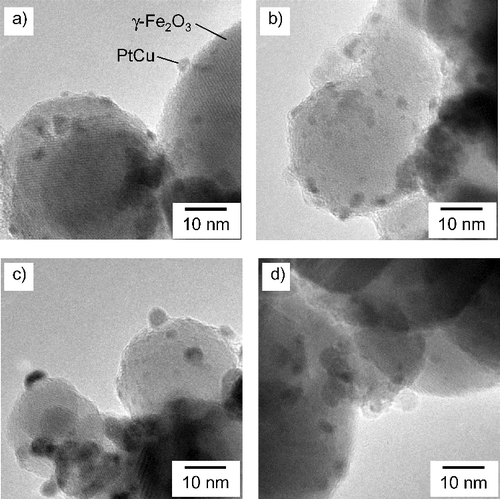
Table 2. Composition of PtCu nanoparticles determined from results (wt.%).
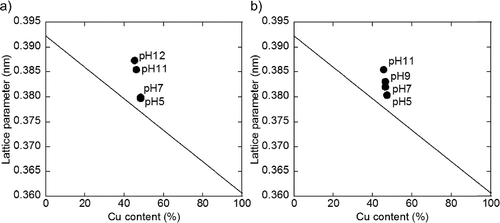
Figure 6. Cu composition in PtCu alloy particle as a function of metal ion content in solution for (a) carbon and (b) γ-Fe2O3 supports (circle denotes Pt ion and square denotes Cu ion).
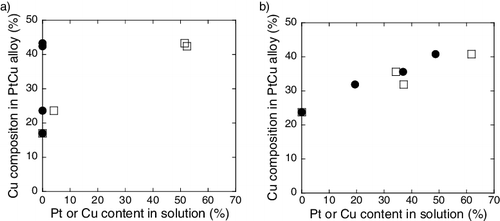
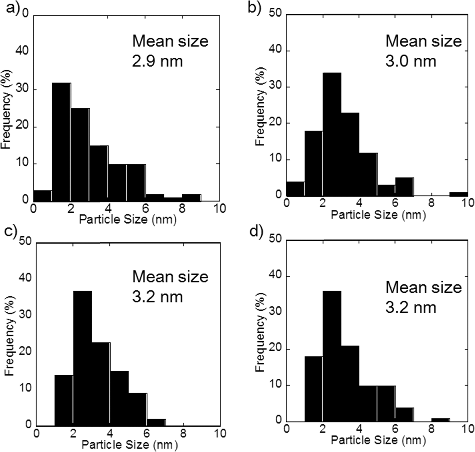
Figure A1. Particle size distribution of PtCu nanoparticles supported on carbon at (a) pH 5, (b) pH 7, (c) pH 11, and (d) pH 12.
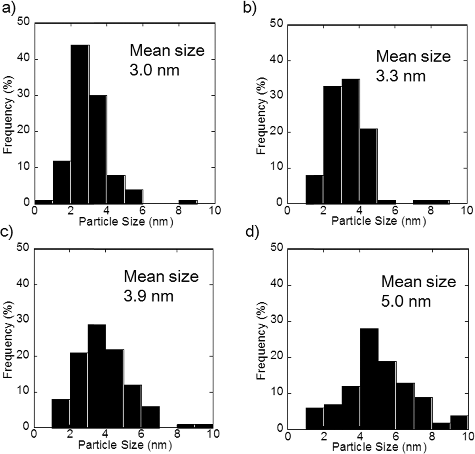
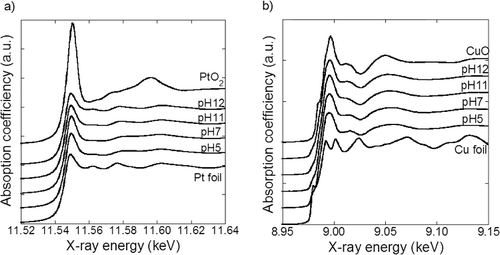
Table B1. Chemical states of Pt and Cu calculated from linear combination fit of XANES spectra.