Figures & data
Figure 2. Effect of initial nitric acid concentration on Me2-CA-BTP/SiO2-P adsorption abilities towards Pd(II) (adsorbent: 0.1 g, solution: 5 cm3, metal: 20 mmol/dm3, temperature: 298 K, contact time: 24 h, shaking speed: 120 rpm).
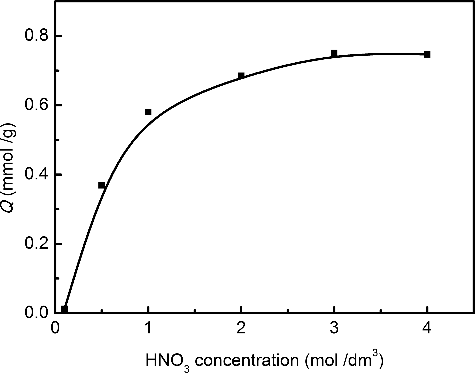
Figure 3. Adsorption kinetics and pseudo-first-order and pseudo-second-order models of Pd(II) on Me2-CA-BTP/SiO2-P in 3.0 mol/dm3 HNO3 solution (adsorbent: 0.1 g, solution: 5 cm3, [Pd]: 20 mmol/dm3, temperature: 298 K, shaking speed: 120 rpm).
![Figure 3. Adsorption kinetics and pseudo-first-order and pseudo-second-order models of Pd(II) on Me2-CA-BTP/SiO2-P in 3.0 mol/dm3 HNO3 solution (adsorbent: 0.1 g, solution: 5 cm3, [Pd]: 20 mmol/dm3, temperature: 298 K, shaking speed: 120 rpm).](/cms/asset/c535ccfe-90bb-41a3-b704-a7aaca03cbb5/tnst_a_1298481_f0003_oc.jpg)
Table 1. Fitted parameters of pseudo-first-order model and pseudo-second-order model for Pd(II) adsorption.
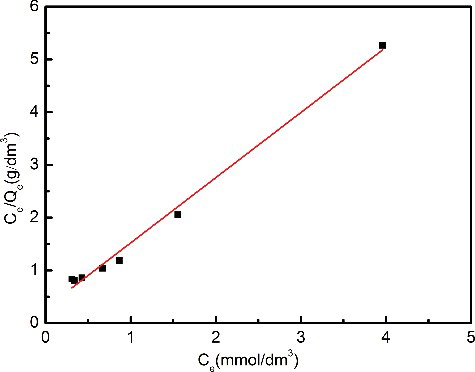
Figure 4. Adsorption isotherm of Pd(II) on Me2-CA-BTP/SiO2-P in 3.0 mol/dm3 HNO3 solution (adsorbent: 0.1 g, solution: 5 cm3, contact time: 24 h, temperature: 298 K, shaking speed: 120 rpm).
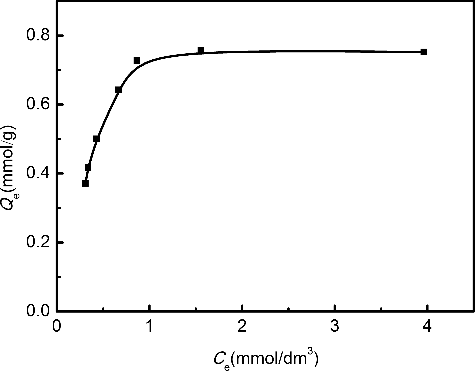
Table 2. Fitted parameters of Langmuir and Freundlich models for Pd (II) adsorption.
Figure 6. Effect of TU concentration on desorption of Pd(II) from Me2-CA-BTP/SiO2-P adsorbent (adsorption conditions: adsorbent: 0.1 g, solution: 5 cm3, [Pd]: 20 mmol/dm3, nitric acid: 3.0 mol/dm3 HNO3, temperature: 298 K, contact time: 24 h, shaking speed: 120 rpm; desorption conditions: adsorbent: 0.1 g, solution: 5 cm3, temperature: 298 K, contact time: 24 h, shaking speed: 120 rpm).
![Figure 6. Effect of TU concentration on desorption of Pd(II) from Me2-CA-BTP/SiO2-P adsorbent (adsorption conditions: adsorbent: 0.1 g, solution: 5 cm3, [Pd]: 20 mmol/dm3, nitric acid: 3.0 mol/dm3 HNO3, temperature: 298 K, contact time: 24 h, shaking speed: 120 rpm; desorption conditions: adsorbent: 0.1 g, solution: 5 cm3, temperature: 298 K, contact time: 24 h, shaking speed: 120 rpm).](/cms/asset/4c5ed2db-062b-4c5b-bda6-a1cd07d4e224/tnst_a_1298481_f0006_b.gif)
Figure 7. Distribution ratios of typical HLLW elements at different concentrations of HNO3 (adsorption conditions: adsorbent: 0.1 g, solution: 5 cm3, 241Am(III): 800 Bq/L, metal: 1 mmol/dm3, temperature: 298 K, contact time: 24 h, shaking speed: 120 rpm).
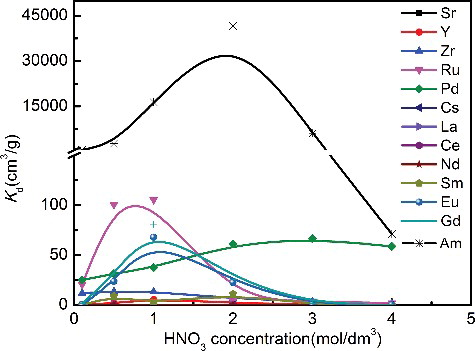
Table 3. Separation factor (SF) between Pd(II) and some typical FP ions.
Figure 8. Effect of contact time with nitric acid solution on the adsorption of Pd(II) onto Me2-CA-BTP/SiO2-P. (adsorption conditions: adsorbent: 0.1 g, solution: 5 cm3, [Pd]: 20 mmol/dm3, temperature: 298 K, contact time: 24 h, shaking speed: 120 rpm).
![Figure 8. Effect of contact time with nitric acid solution on the adsorption of Pd(II) onto Me2-CA-BTP/SiO2-P. (adsorption conditions: adsorbent: 0.1 g, solution: 5 cm3, [Pd]: 20 mmol/dm3, temperature: 298 K, contact time: 24 h, shaking speed: 120 rpm).](/cms/asset/2622495c-3469-4699-9181-fc0898c531d8/tnst_a_1298481_f0008_oc.jpg)

![Figure 9. Effect of absorbed dose on Me2-CA-BTP/SiO2-P(dry state) adsorption towards Pd(II) (adsorption conditions: adsorbent: 0.1 g, solution: 5 cm3, [Pd]: 20 mmol/dm3, nitric acid: 3.0 mol/dm3 HNO3, temperature: 298 K, contact time: 24 h, shaking speed: 120 rpm).](/cms/asset/5ea2a04f-7d37-49fa-9a46-64d145edfeb4/tnst_a_1298481_f0009_b.gif)
![Figure 10. Effect of absorbed dose on Me2-CA-BTP/SiO2-P (in nitric acid) adsorption towards Pd(II) (adsorption conditions: adsorbent: 0.1 g, solution: 5 cm3, [Pd]: 20 mmol/dm3, temperature: 298 K, contact time: 24 h, shaking speed: 120 rpm).](/cms/asset/fea11b58-20f1-4ce3-b86f-44b240a7e25e/tnst_a_1298481_f0010_oc.jpg)