Figures & data
Figure 3. XPS spectra of (a) total survey scan, (b) C 1 s of GO-DTPAA, (c) O1s of GO-DTPAA, (d) N 1 s of GO-DTPAA, and (e) U 4f of GO-DTPAA-U.
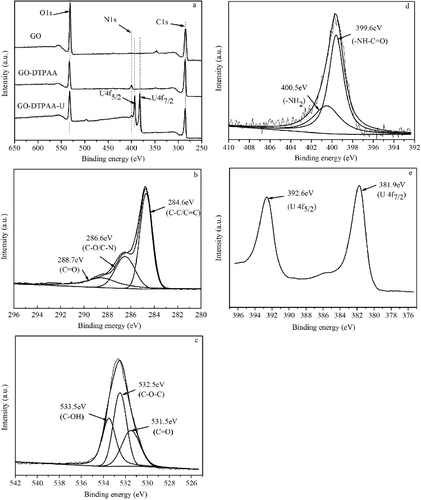
Table 1. Composition of GO, GO-DTPAA, and GO-DTPAA-U
Figure 5. SEM images of (a) GO-DTPAA adsorbed uranium and (b) the EDS image of GO-DTPAA adsorbed with U(VI).
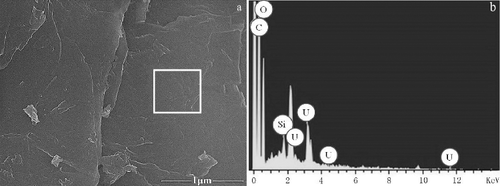
Figure 7. Effects of pH on U(VI) adsorption onto GO and GO-DTPAA. (C[U(VI)] = 50 mg·L−1 and T = 25 °C).
![Figure 7. Effects of pH on U(VI) adsorption onto GO and GO-DTPAA. (C[U(VI)] = 50 mg·L−1 and T = 25 °C).](/cms/asset/035ed8d0-bb6a-4e67-bfd2-74812fc30ec4/tnst_a_1439415_f0007_b.gif)
Figure 8. Effects of time on U(VI) adsorption onto GO and GO-DTPAA. (C[U(VI)] = 50 mg·L−1, pH = 6.5 and T = 25 °C).
![Figure 8. Effects of time on U(VI) adsorption onto GO and GO-DTPAA. (C[U(VI)] = 50 mg·L−1, pH = 6.5 and T = 25 °C).](/cms/asset/58b2256b-b99e-4dfc-97ec-42c10f71f2a9/tnst_a_1439415_f0008_b.gif)
Figure 9. Effects of initial U(VI) concentration on U(VI) adsorption onto GO and GO-DTPAA. (pH = 6.5 and T = 25 °C).
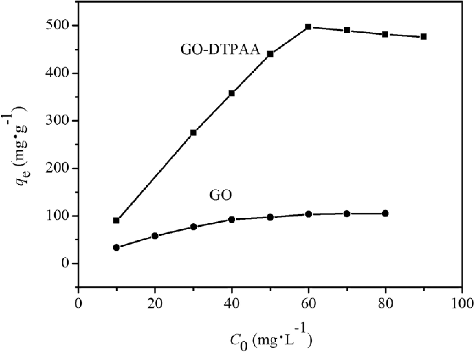
Table 2. Adsorption kinetic parameters of uranium by GO and GO-DTPAA
Table 3. Langmuir, Freundlich, and D–R isotherm parameters of pristine GO and GO-DTPAA
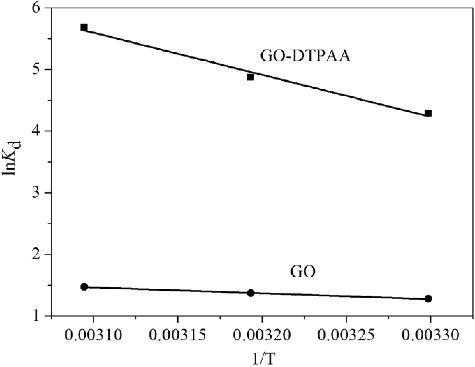
Table 4. Thermodynamic parameters for U(VI) adsorption onto GO and GO-DTPAA
Table 5. Comparison of U(VI) adsorption capacity by different sorbents