Figures & data
Table 1. Values Λ[ρ] for some simple spherical two-electron trial densities ρ(r) in three dimensions (N = 2, D = 3), obtained numerically from Equations (EquationA9(A9)
(A9) )–(EquationA11
(A11)
(A11) ) of Appendix A. In the last two rows, we consider densities with compact support: ‘droplet’ corresponds to the case of a sphere of uniform density [Citation24], and the density proportional to r−3 [Citation11] has been evaluated for R1 = 103 and R2 = 105. [Atomic units are used, where r is a dimensionless radial coordinate.]
Table 2. Exact values for various values of ε < 0, compared with the first-order expansion (see Section 3.3).
Table 3. For different values of N, we compare the Λ[ρ] obtained from atomic densities (values from [Citation21]) with the ones obtained from spherical droplets of uniform density (values from [Citation24]).
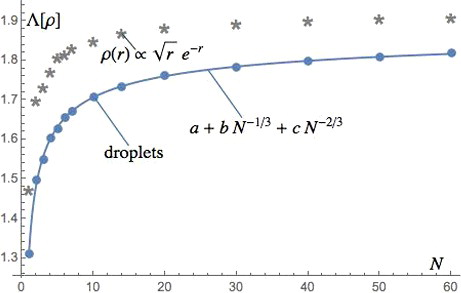
Figure 1. Values of Λ[ρ] for the fixed density profile ρ(r)∝r1/2e−r compared with those for spheres of uniform density (droplets) as a function of the particle number N. The values for ρ(r)∝r1/2e−r are significantly higher than those for uniform densities. The size extrapolation for uniform densities is also shown, where the fitting parameters are a = 1.918, b = −0.3253, c = −0.2791.
![Figure 1. Values of Λ[ρ] for the fixed density profile ρ(r)∝r1/2e−r compared with those for spheres of uniform density (droplets) as a function of the particle number N. The values for ρ(r)∝r1/2e−r are significantly higher than those for uniform densities. The size extrapolation for uniform densities is also shown, where the fitting parameters are a = 1.918, b = −0.3253, c = −0.2791.](/cms/asset/0daf42d6-399f-4583-b093-af74476f4ca4/tmph_a_1136440_f0001_oc.jpg)