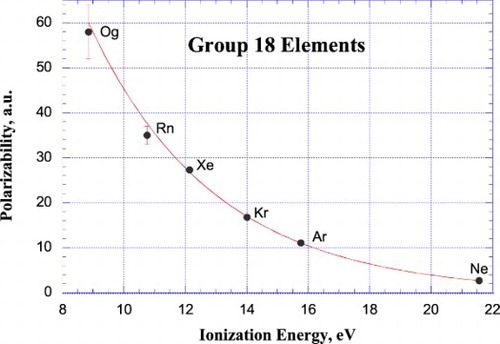
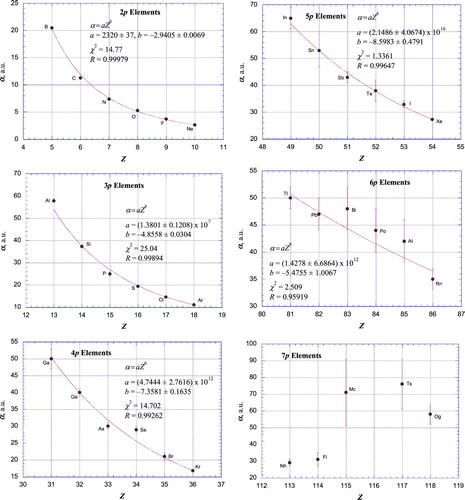
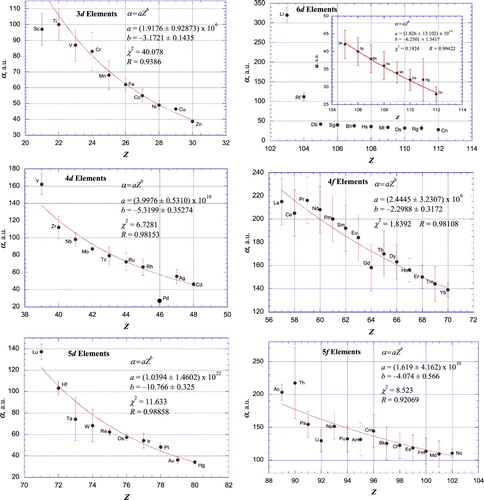
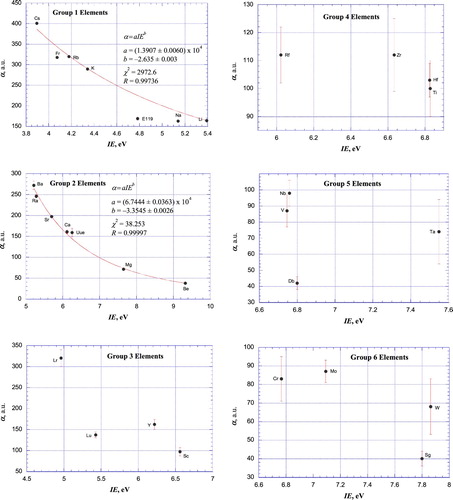
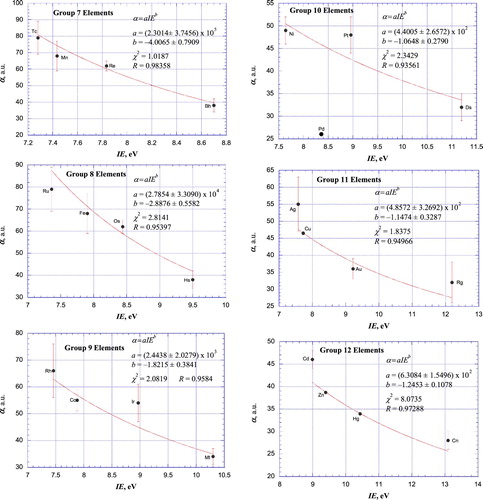
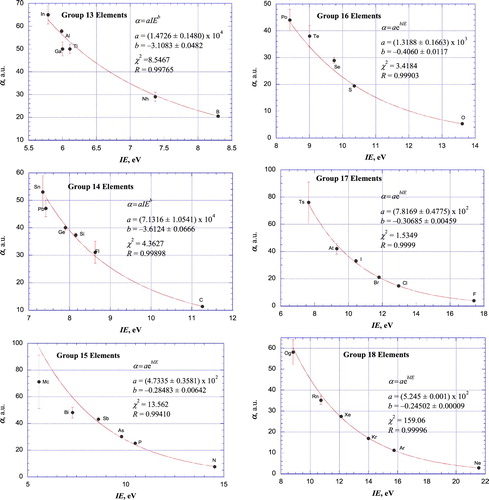
Figures & data
Table 1. Static scalar dipole polarizabilities (in atomic units) for neutral atoms.