Figures & data
Figure 1. A two-site associating chain molecule comprising five tangentially bonded spherical segments and two association sites labelled A and B. The position of the centre of mass of the molecule is indicated by , and the displacement site vectors by
and
, which are function of the molecular orientation and conformation
. The distance between two segments α and β of the molecule at position
is represented by
.
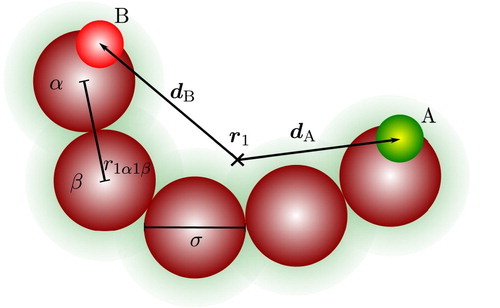
Figure 2. Constraints of TPT1 [Citation28] restrict the following bonding schemes: (a) the repulsive cores of two associating molecules prevent a third core participating in bonding; (b) a site may participate in one bond only; (c) double-bonding between any two segments is not permitted.
![Figure 2. Constraints of TPT1 [Citation28] restrict the following bonding schemes: (a) the repulsive cores of two associating molecules prevent a third core participating in bonding; (b) a site may participate in one bond only; (c) double-bonding between any two segments is not permitted.](/cms/asset/d39392e1-b6ee-4945-98e6-8faf4d8be823/tmph_a_1671619_f0002_oc.jpg)
Figure 3. Examples of association aggregates formed in a spherical model fluid of molecules shown in (a) with three association sites (labelled A, B, C). The check marks and crosses indicate whether the aggregate type is accounted for by the theory presented in our current work or not: (a) monomer species; (b) open branched chain; (c) intermolecular ring bonded through A–B bonds only; (d) intermolecular ring bonded through A–B bonds only, with further branching; (e) A–B intermolecular ring associated to a B–C intermolecular ring; and (f) intermolecular ring involving more than one type of site–site interaction.
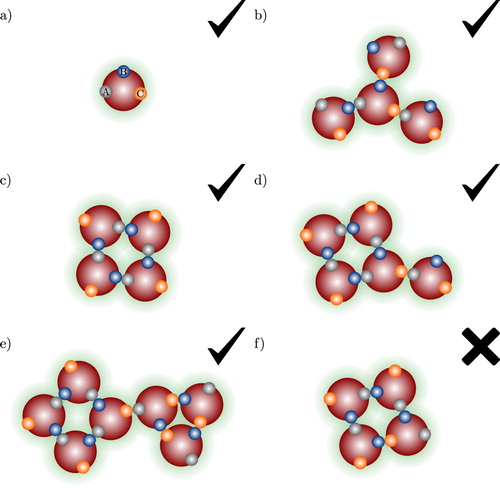
Figure 4. Examples of the association aggregates that can be found in a fluid of chain molecules shown in (a) with multiple association sites, A, B, C,….The check marks and crosses indicate whether the aggregate type is accounted for by the theory presented in our current work or not: (a) monomer species; (b) intramolecular ring; (c) open chain aggregate; (d) intermolecular ring containing only one type of site–site bond (B–C); (e) intramolecular ring with a C–E bond associated to an A–E intramolecular ring; (f) branched A–E intramolecular ring; (g) molecule involved in more than one ring; and (h) ring involving more than one type of site–site interaction.
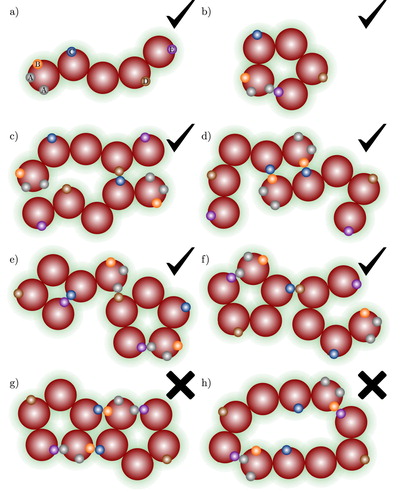
Table 1. Like and unlike interaction parameters for the model systems, where 
 are the component indexes (1 for the chain and 2 for the sphere),
are the component indexes (1 for the chain and 2 for the sphere), 
 is the segment diameter,
is the segment diameter, 
 and
and 
 are the repulsive and attractive exponents of the Mie potential, respectively, and
are the repulsive and attractive exponents of the Mie potential, respectively, and 
 is the depth of the potential well.
is the depth of the potential well.
Figure 5. Fluid-phase coexistence properties of two-site associating chain fluids (comprising m= 5 segments) in different associating schemes: (a) vapour pressure; (b) Clausius–Clapeyron representation; (c) saturated densities with inset of saturated volumes; and (d) vaporisation enthalpy. The reduced pressure is defined by , the reduced temperature by
, the packing fraction by
, the reduced volume by
, and the reduced enthalpy by
. The dashed black curves correspond to calculations for a non-associating fluid, the continuous black curves to an associating fluid with
, the continuous green curves to
, the continuous blue curves to
, and the continuous pink curves to
.
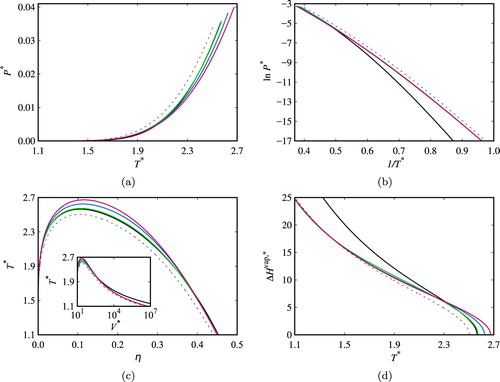
Figure 6. Fractions of molecules in each bonding state as a function of temperature and packing fraction
: (a–c) in a low-density phase (
) as a function of temperature; (d–f) in a high-density phase (
) as a function of temperature; and (g–i) at fixed temperature
as a function of packing fraction. The fraction of linear aggregates is given by the width of the solid area, the fraction of molecules with intramolecular HB by the width of the square-patterned area, and the fraction of monomers by the width of the white area. The subfigures to the left refer to the associating fluid in the absence of intramolecular HB (
), the subfigures in the centre to the model with
, and the subfigures to the right to
.
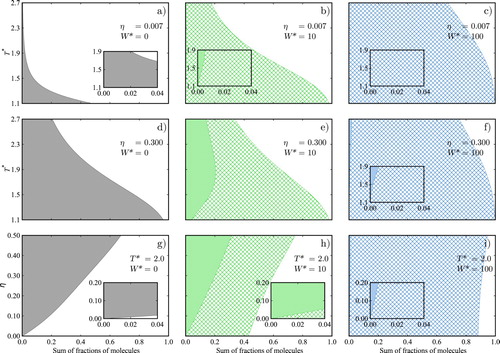
Figure 7. Vapour–liquid equilibria of pure chain fluids with ,
, and
segments with interaction parameters given in Table : (a) vapour pressures; (b) Clausius–Clapeyron representation; (c) saturated densities; and (d) vaporisation enthalpies. The dashed curves correspond to non-associating systems, the continuous curves to systems with intermolecular association only, the long-dashed curves to those with intramolecular association with
, and the dash-dotted curves to those with intramolecular association with
.
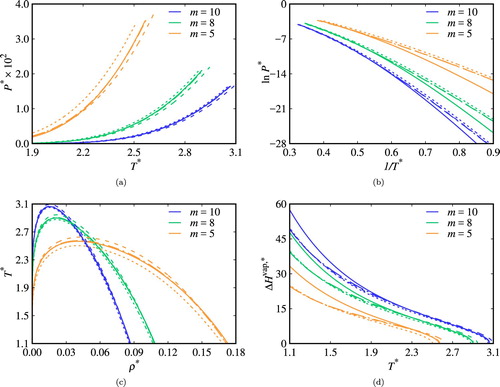
Figure 8. Temperature vs. (a) fraction of monomers
, (b) fraction
of molecules in open-chain aggregates, and (c) fraction
of molecules in intramolecular rings, along the saturated-vapour and liquid states for chain fluids of
,
, and
segments. The continuous curves correspond to models with
, the dash-dotted curves to
, and the long-dashed curves to
.
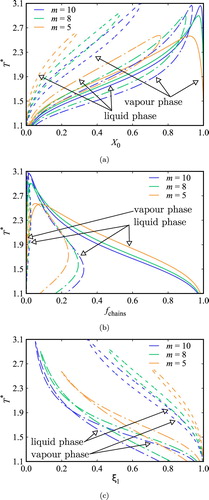
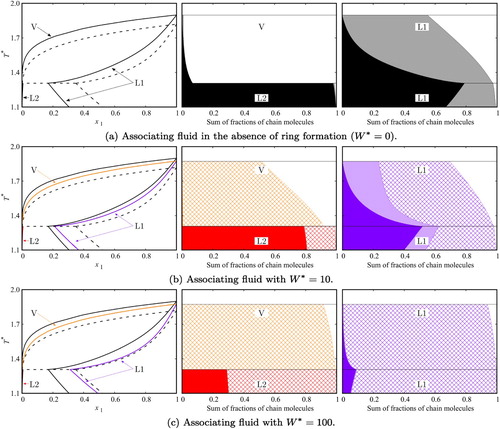
Figure 9. Fluid-phase equilibria and distribution of bonded states along the phase boundaries for a binary mixture of two-site (A and B) associating chains of m=5 segments (component 1) and spheres with one association site A (component 2), which can bond to site B in component 1 only (no AA association is allowed) at a constant pressure . The phase boundaries are labelled as V for the saturated vapour, and L1 and L2 for the saturated liquid phases rich in component 1 and 2, respectively. The black dashed curves in (a), (b), and (c) correspond to calculations for non-associating chains, the black continuous curves to associating chains with
, and the coloured curves in (b) and (c) to associating chains with
and
, respectively. The fractions of chain molecules in the possible bonding states along the saturation curves are represented in the middle and right-hand side panels. For a given temperature, the fraction of cross-associated molecules of component 1 is represented by the width of the dark-colour solid area, the fraction of self-associated molecules by the width of the light-colour solid area, the fraction of chains with intramolecular HB by the width of the square-patterned area, and the fraction of non-bonded (free) chains by the width of the white area. (a) Associating fluid in the absence of ring formation (
); (b) associating fluid with
; and (c) associating fluid with
.