Figures & data
Figure 1. Experimentally observed (red) and theoretically predicted (blue, green) spectrum of trans-MC in cyclohexane. Both the highest observed experimental absorption and highest predicted oscillator strength were normalised to 1. Blue lines denote two excitations with ππ* character and the green line denotes a darker excitation with nπ* character. The orbitals that are primarily involved in the lowest energy ππ* and nπ* transitions are displayed inset.
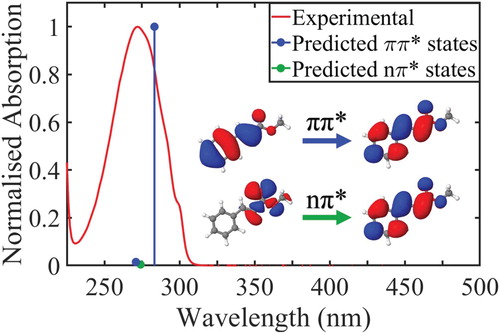
Figure 2. top: TR-IY and TR-PES transients for trans-MC photoexcited at 294 nm (around the 11ππ* origin), and probed with 200 nm. The polarisations of the pump and probe beams were parallel with respect to each other in both TR-IY and TR-PES experiments so that the results were comparable. bottom: TKER spectra obtained following photoexcitation of trans-MC at 294 nm and at a pump–probe delay of Δt = −5, 0 and 10 ps. Inset: PES-VMI at Δt = 0. Black arrow shows the value for TKERmax.
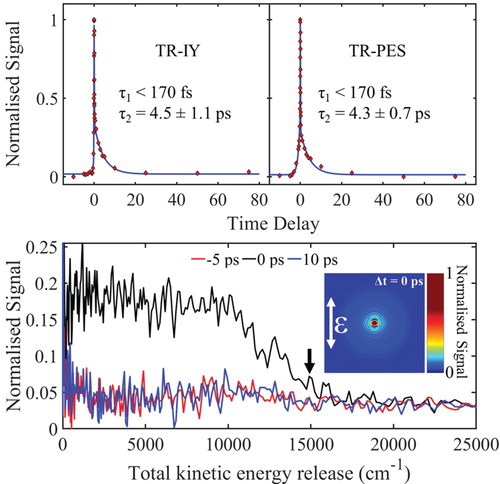
Figure 3. top: Steady-state UV-Vis absorption spectra corresponding to the non-irradiated (red curve) and after 30 min of constant irradiation (blue curve) of trans-MC at 280 nm. bottom: 1H NMR spectra of trans-MC in deuterated cyclohexane before (blue curve) and after 30 min (red curve) of irradiation at 280 nm.
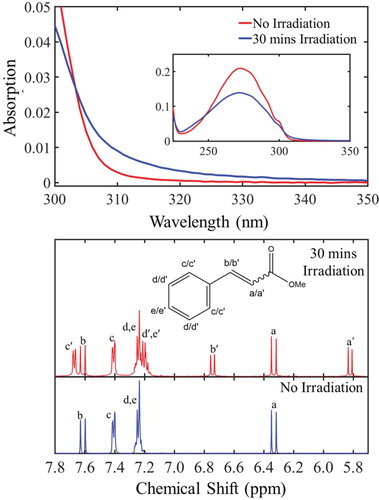
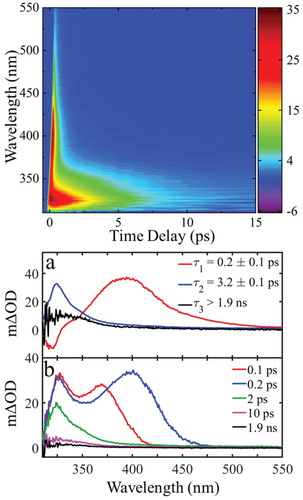
Figure 4. top: False colour map showing TAS of trans-MC in cyclohexane with λpump = 280 nm bottom: (a) Decay associated spectra (DAS) extracted from global fit. The DAS amplitude corresponding to τ3 (DAS3) is multiplied by 5 to achieve a better illustration. (b) TAS at selected pump-probe time delays. These plots correspond to vertical slices through the false colour heat map at the selected time delays.