Figures & data
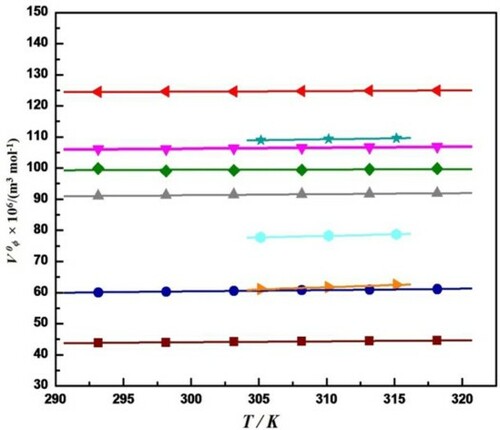
Table 1. Literature reported data of limiting apparent molar volume, V 0ϕ, for different amino acids in various drugs at different temperatures.
Figure 1. Plot of Limiting apparent molar volume, V0ϕ, versus temperature, T, for different amino acid in aqueous solution of metformin hydrochloride at different concentrations (a) ![]()
 , L-threonine in 0.09 mol kg−1 metformin hydrochloride [Citation20], (c)
, L-threonine in 0.09 mol kg−1 metformin hydrochloride [Citation20], (c)  , L-glutamine in 0.07 mol kg−1 metformin hydrochloride [Citation21], and (c)
, L-glutamine in 0.07 mol kg−1 metformin hydrochloride [Citation21], and (c)  , L-proline in 0.10 mol kg−1 metformin hydrochloride [Citation22].
, L-proline in 0.10 mol kg−1 metformin hydrochloride [Citation22].![Figure 1. Plot of Limiting apparent molar volume, V0ϕ, versus temperature, T, for different amino acid in aqueous solution of metformin hydrochloride at different concentrations (a) Display full size, L-serine in 0.09 mol kg−1 Metformin hydrochloride [Citation20], (b) Display full size, L-threonine in 0.09 mol kg−1 metformin hydrochloride [Citation20], (c) Display full size, L-histidine in 0.07 mol kg−1 metformin hydrochloride [Citation21], (d) Display full size, L-glutamine in 0.07 mol kg−1 metformin hydrochloride [Citation21], and (c) Display full size, L-proline in 0.10 mol kg−1 metformin hydrochloride [Citation22].](/cms/asset/8810cdb4-b80e-488f-93cc-dd2dff799ff3/tmph_a_1992029_f0001_oc.jpg)
Figure 2. Plot of Limiting apparent molar volume, V0ϕ, versus temperature, T, for different amino acid in aqueous solution of Streptomycin sulphate at different concentrations (a) ![]()
 , L-alanine in 01% Streptomycin sulphate [Citation2], (c)
, L-alanine in 01% Streptomycin sulphate [Citation2], (c)  , L-isoleucine in 1% Streptomycin sulphate [Citation2], and (e)
, L-isoleucine in 1% Streptomycin sulphate [Citation2], and (e)  , L-histidine in 1% Streptomycin sulphate [Citation23], (f)
, L-histidine in 1% Streptomycin sulphate [Citation23], (f)  , L-arginine in 1% Streptomycin sulphate [Citation23], (g)
, L-arginine in 1% Streptomycin sulphate [Citation23], (g) ![Figure 2. Plot of Limiting apparent molar volume, V0ϕ, versus temperature, T, for different amino acid in aqueous solution of Streptomycin sulphate at different concentrations (a) Display full size, Glycine in 1% Streptomycin sulphate [Citation2], (b) Display full size, L-alanine in 01% Streptomycin sulphate [Citation2], (c) Display full size, L-valine in 1% Streptomycin sulphate [Citation2], (d) Display full size, L-isoleucine in 1% Streptomycin sulphate [Citation2], and (e) Display full size, L-histidine in 1% Streptomycin sulphate [Citation23], (f) Display full size, L-arginine in 1% Streptomycin sulphate [Citation23], (g) Display full size, L-serine in 0.02 mol kg−1 Streptomycin sulphate [Citation24], (h) Display full size,L-threonine in 0.02 mol kg−1 Streptomycin sulphate [Citation24], and (i) Display full size, L-leucine in 0.02 mol kg−1 Streptomycin sulphate [Citation24].](/cms/asset/8b42f749-a38a-4fd8-a71b-b9e0cc0ccc1f/tmph_a_1992029_f0002_oc.jpg)
Table 2. Literature reported data of limiting apparent molar volume of transfer, ΔtrV0ϕ, for different amino acids in various drugs at different temperatures.
Table 3. Literature reported data of limiting apparent exapansivity, E 0ϕ, for different amino acids in various drugs at different temperatures.
Table 4. Literature reported data of Hepler’s constant, (∂E0ϕ /∂T)P, for different amino acids in various drugs.
Table 5. Literature reported data of Hydration number, nH for different amino acids in various drugs at different temperatures.
Table 6. Literature reported data of Viscosity B-cofficient, B, for different amino acids in various drugs at different temperatures.
