Figures & data
Figure 2. Reaction energy profile for the reaction of cholesterol and 1O2 to produce 5-hydroperoxycholesterol (see Figure ). Molecular structures of transition states and intermediates are given in Scheme 1 and Figure . All values are relative to the previous step.
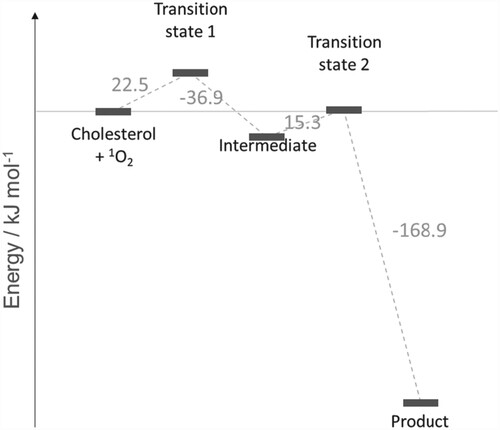
Figure 3. Reaction energy profile for the reaction of cholesterol and 1O2 to produce R-6-hydroperoxycholesterol (see Figure ). Molecular structures of transition states and intermediates are given in Scheme 2 and Figure . All values are relative to the previous step.
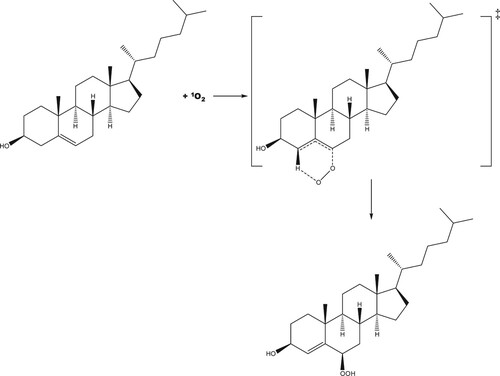
Figure 4. Reaction energy profile for the reaction of cholesterol and 1O2 to produce S-6-hydroperoxycholesterol (see Figure ). Molecular structures of transition states and intermediates are given in Scheme 3 and Figure . All values are relative to the previous step.
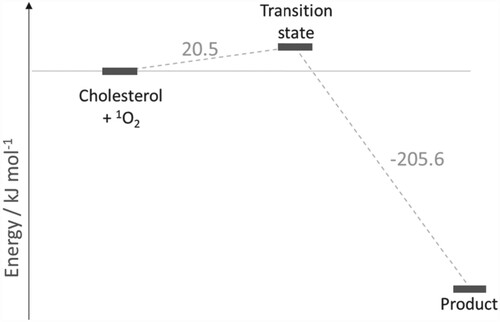
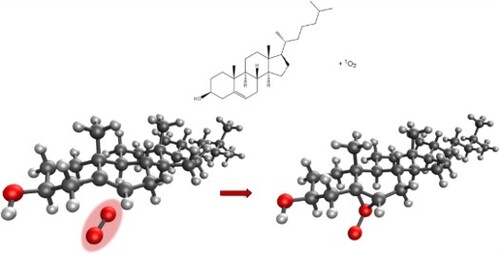
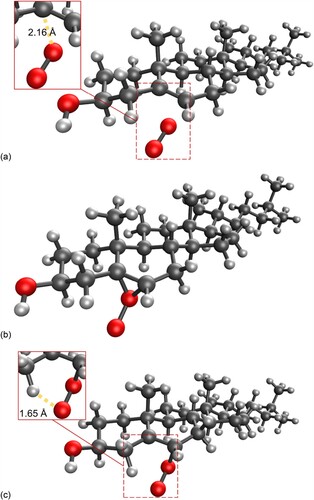
Figure 5. (a) Molecular structure of the first transition state for the addition of singlet oxygen to cholesterol at the 5 position; (b) Epoxide-like intermediate; (c) second transition state, leading to the formation of 5-hydroperoxycholesterol.
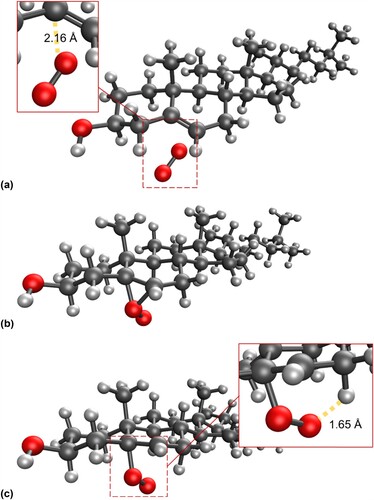
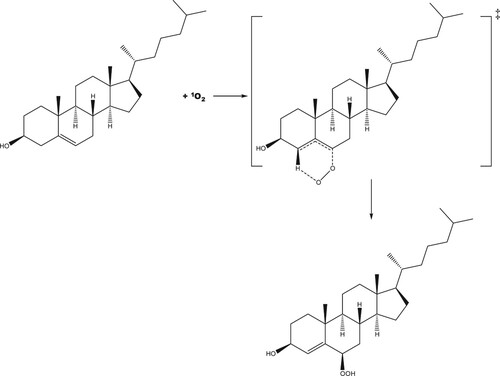
Figure 6. Molecular structure of the concerted transition state for the addition of singlet oxygen to cholesterol at the 6 position to form the R-6-hydroperoxycholesterol.
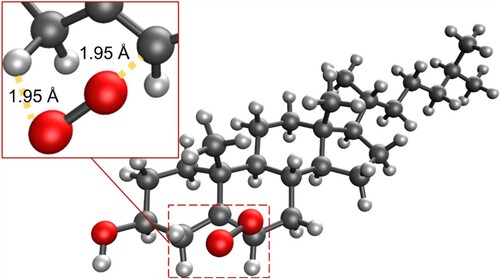
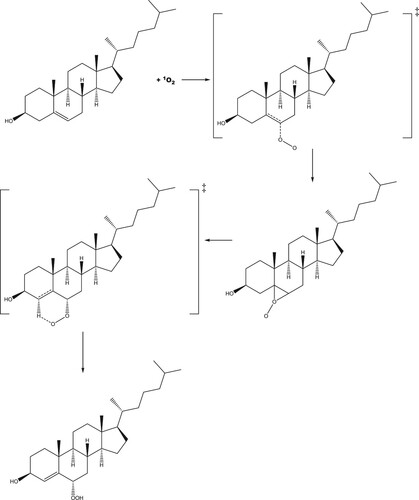
Figure 7. (a) Molecular structure of the first transition state for the addition of singlet oxygen to cholesterol at the 6 position; (b) Epoxide-like intermediate; (c) second transition state, leading to the formation of S-6-hydroperoxycholesterol.
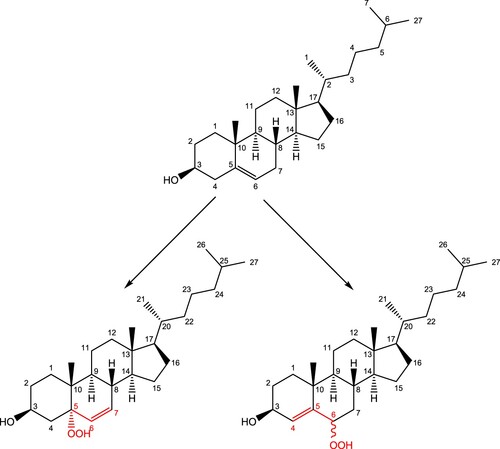
Scheme 1. Proposed reaction scheme for the production of 5-hydroperoxycholesterol via the ‘ene' reaction.
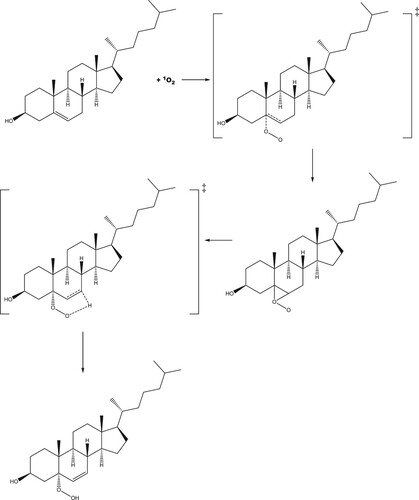
Table 1. Gibbs free energy values for the different reactions (kJ mol−1). All values are relative to the reactants.
Table 2. Calculated rate constants for the rate limiting steps for each of the products considered in this study. All calculated rate constants were calculated at a temperature of 298.15 K in the condensed phase.