Figures & data
Table 1. Average of absolute rate coefficients kabs of reactions (1)–(5) are compared with calculated collision rate coefficients kADO, resulting in the efficiency ϕ = kabs/kADO. Errors correspond to the standard deviation of all measuremts. The energy release of each reaction in eV was calculated as the CCSD/aug-cc-pVDZ level of theory single point energy combined of the MP2/aug-cc-pVDZ structures and zero-point energies.
Table 2. Comparison of experimental [Citation18] and calculated proton affinities of argon, nitrogen, and acetone. The calculated values contain MP2/aug-cc-pVDZ, and CCSD/aug-cc-pVDZ optimised values including their respective thermal enthalpy corrections. Furthermore, CCSD/aug-cc-pVDZ single-point energies using MP2/aug-cc-pVDZ optimised structures and thermal enthalpy corrections are listed.
Figure 1. Reaction kinetics of (a) N2H+ and (b) ArH+ with acetone at a backing pressure of 1.9 × 10−8 mbar and 1.3 × 10−8 mbar, respectively. For (a) and (b) the first 0.15 and 0.8 s were neglected for the fit, respectively, as thermalisation effects conflict with the pseudo-first order reaction model used for fitting the results.
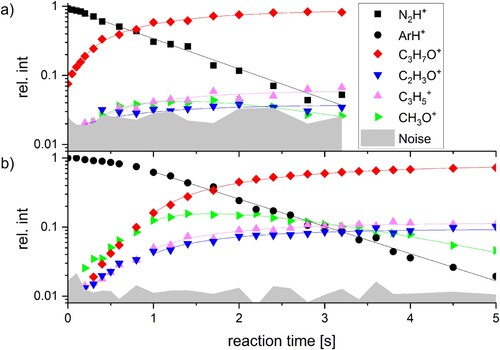
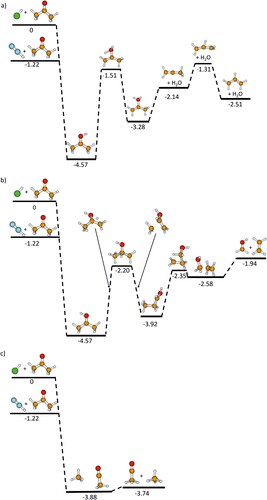
Figure 2. Reaction profiles of PTR from N2H+ and ArH+ to acetone and formation of (a) C3H5+ with loss of H2O; (b) CH2OH+ with loss of C2H4; (c) C2H3O+ with loss of CH4. Structures are calculated at the MP2/augcc-pVDZ level of theory, the energies are calculated using CCSD/aug-cc-pVDZ single-point energies and the zero-point corrections derived from MP2/aug-cc-pVDZ level of theory. The trivial neutrals (Ar or N2) are not shown after the PTR, but their energies were considered.