Figures & data
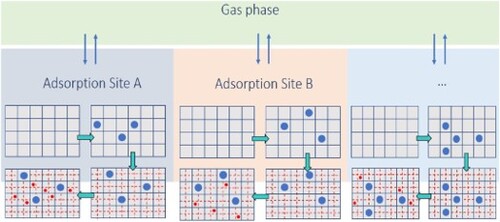
Figure 1. Equilibrium of each of the adsorbed phases with the gas phase in the Segregated Ideal Adsorbed Solution Theory (SIAST) model [Citation22]. Each adsorbed phase is separately in equilibrium with the gas phase. The system is at a constant temperature. The gas phase has a total pressure of and the mole fraction of component i equals
. In the adsorbed phase j, the loading of component i is
.
![Figure 1. Equilibrium of each of the adsorbed phases with the gas phase in the Segregated Ideal Adsorbed Solution Theory (SIAST) model [Citation22]. Each adsorbed phase is separately in equilibrium with the gas phase. The system is at a constant temperature. The gas phase has a total pressure of ptot and the mole fraction of component i equals yi. In the adsorbed phase j, the loading of component i is qi,j.](/cms/asset/e09f224c-2f92-42bc-8ab8-3309d3a5d1e6/tmph_a_2183721_f0001_oc.jpg)
Figure 2. Representation of the adsorbent as a lattice where species of different sizes are adsorbed [Citation34]. (a) The adsorbent is divided into sites. (b) adsorption of N indistinguishable molecules of species 1, represented by the blue circles, is taking place on the lattice. (c) After the adsorption of N molecules of species 1, the remaining lattice sites are further divided into
sites. (d) adsorption of K indistinguishable molecules of species 2 is taking place on the lattice. The blue circles represent species 1 and the smaller red circles represent species 2.
![Figure 2. Representation of the adsorbent as a lattice where species of different sizes are adsorbed [Citation34]. (a) The adsorbent is divided into M1 sites. (b) adsorption of N indistinguishable molecules of species 1, represented by the blue circles, is taking place on the lattice. (c) After the adsorption of N molecules of species 1, the remaining lattice sites are further divided into (nM1−nN) sites. (d) adsorption of K indistinguishable molecules of species 2 is taking place on the lattice. The blue circles represent species 1 and the smaller red circles represent species 2.](/cms/asset/73fcc5b1-9cde-4be8-a62b-817313fa4f57/tmph_a_2183721_f0002_oc.jpg)
Figure 3. Representation of Segregated Explicit Isotherm (SEI) model which accounts for the separate thermodynamic equilibria of the adsorbed phases with the gas phase at different adsorption sites. Each adsorbed phase is separately in thermodynamic equilibrium with the gas phase and it is represented by the adsorption isotherm proposed by Van Assche et al. [Citation34] Equation (Equation23(23)
(23) ). The gas phase has a total pressure of
and the gas phase mole fractions of the components in the mixture are represented by
. In the adsorbed phase j, the loading of the component i is
.
![Figure 3. Representation of Segregated Explicit Isotherm (SEI) model which accounts for the separate thermodynamic equilibria of the adsorbed phases with the gas phase at different adsorption sites. Each adsorbed phase is separately in thermodynamic equilibrium with the gas phase and it is represented by the adsorption isotherm proposed by Van Assche et al. [Citation34] Equation (Equation23(23) qi=(qmax,1kipi)[∏m=1iαm]β(23) ). The gas phase has a total pressure of ptot and the gas phase mole fractions of the components in the mixture are represented by yi. In the adsorbed phase j, the loading of the component i is qi,j.](/cms/asset/af075c80-ae2a-435e-b1af-fe731df8f3ad/tmph_a_2183721_f0003_oc.jpg)
Table 1. Input parameters for the simulation of the breakthrough curve (catalyst particle density, , bed voidage
, interstitial velocity at the inlet,
, and total pressure
) for both cases.
Table 2. Mass transfer coefficients for the components in both the case studies.
Figure 4. Pure component adsorption isotherms of and
in MOR-type zeolite at 300K. Pure component adsorbed loadings calculated using GCMC for the pressure range
Pa are fitted to the dual-site Langmuir isotherms. The empty circles represent GCMC simulation data and the solid lines are the dual-site Langmuir isotherms fitted to these data sets. The results obtained in this work are in excellent agreement with the data published by Swisher et al. [Citation22].
![Figure 4. Pure component adsorption isotherms of CO2 and C3 in MOR-type zeolite at 300K. Pure component adsorbed loadings calculated using GCMC for the pressure range (100−5⋅1010) Pa are fitted to the dual-site Langmuir isotherms. The empty circles represent GCMC simulation data and the solid lines are the dual-site Langmuir isotherms fitted to these data sets. The results obtained in this work are in excellent agreement with the data published by Swisher et al. [Citation22].](/cms/asset/ccf59a01-6be6-4f00-8970-16741ce6f100/tmph_a_2183721_f0004_oc.jpg)
Table 3. Fitted parameters for the adsorption of pure and
in MOR-type zeolite at 300 K.
Figure 5. Snapshots of adsorption of pure CO and C
molecules in MOR-type zeolite at 300K using GCMC simulations. (a) adsorption of
at
Pa, (b) adsorption of
at
Pa, (c) adsorption of
at
Pa and (d) adsorption of
at
Pa. Site 1 represents the smaller pockets and site 2 represents the larger pockets inside MOR-type zeolite
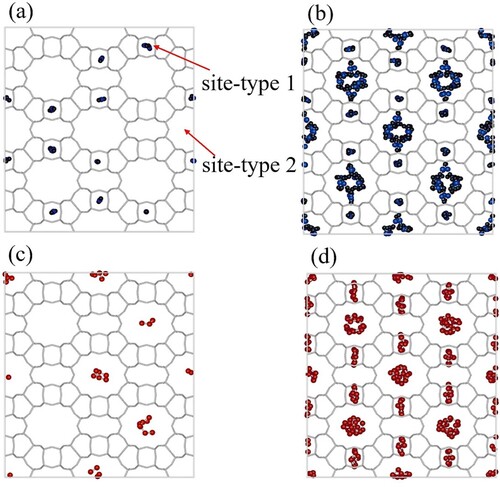
Figure 6. Adsorption isotherms of an equimolar mixture and
(50:50) in MOR-type zeolite at 300K. A comparison is made between the adsorbed loadings calculated using GCMC, IAST, SIAST and SEI. The pressure range
Pa is considered for the calculations performed using IAST, SIAST and SEI. For GCMC simulations, the pressure range is
Pa. Cross marks represent GCMC calculations, triangles are used for IAST, circles for SIAST and squares for SEI.
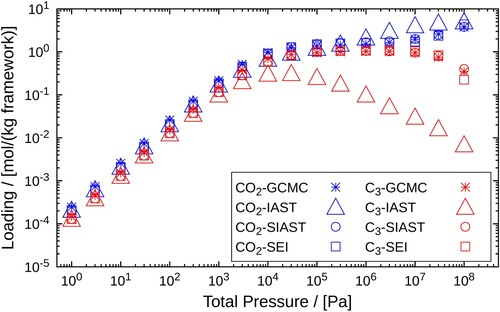
Figure 7. Pure component adsorption isotherms of and
in MFI-type zeolite at 400K. Pure component adsorbed loadings calculated using GCMC for the pressure range
Pa are fitted using the dual-site Langmuir isotherms. The empty circles represent GCMC simulation data and the solid lines are the dual-site Langmuir isotherms fitted to these data sets.
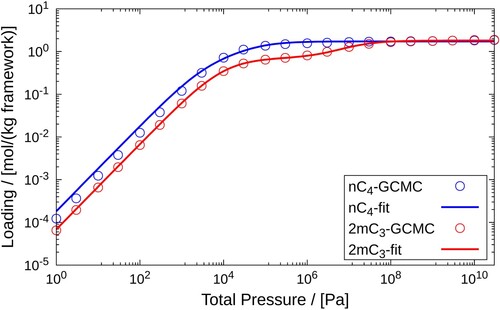
Table 4. Fitted parameters for the adsorption of pure and
in MFI-type zeolite at 400K.
Figure 8. Typical snapshots of adsorption of pure C and 2mC
molecules in MFI-type zeolite at 400K using GCMC simulations. (a) adsorption of
at
Pa, (b) adsorption of
at
Pa, (c) adsorption of
at
Pa and (d) adsorption of
at
Pa. Site 1 represents the intersections and site 2 represent the channels present inside the MFI-type zeolite.
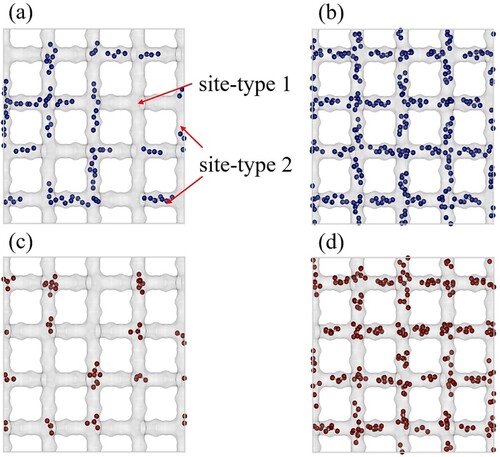
Figure 9. Adsorption isotherms of an equimolar mixture and
in MFI-type zeolite at 400K. A comparison is drawn between the adsorbed loadings calculated using GCMC, IAST, SIAST and SEI. The pressure range
Pa is considered for the calculations performed using IAST, SIAST and SEI. For GCMC simulations, the pressure range is
Pa. Cross marks represent GCMC calculations, triangles are used for IAST, circles for SIAST and squares for SEI.
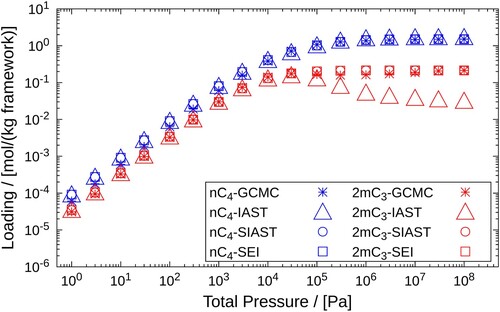
Figure 10. Comparison of breakthrough curves obtained on implementing IAST, SIAST and SEI to the breakthrough curve model. Separation of (a) -
mixture using MOR-type zeolite at 300K and
Pa, (b)
-
mixture using MFI-type zeolite at 400K and
Pa. Each component constitutes
of the mixture in the gas phase. The remaining amount is a non adsorbing carrier gas (helium). Solid lines represent the implementation of SEI. Dashed-dotted lines are used for the SIAST model and dashed lines are used for the IAST model.
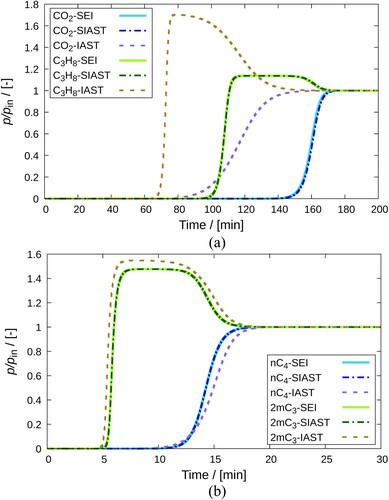
Table 5. Variation in simulation run time for the breakthrough curve model on implementing SEI, SIAST and IAST.
