Figures & data
Figure 1. Electronic tagging photodissociation spectra of He-Phen2+ recorded at different levels of laser power. The Y-axis shows the absolute depletion value, 1-Ni/N0.
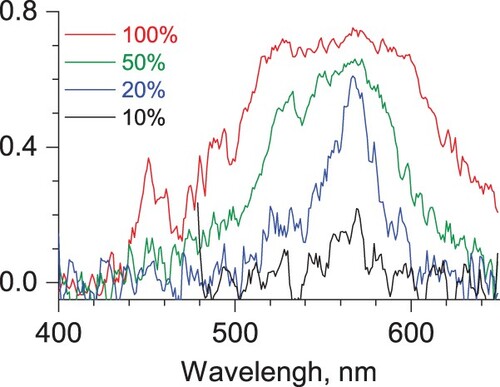
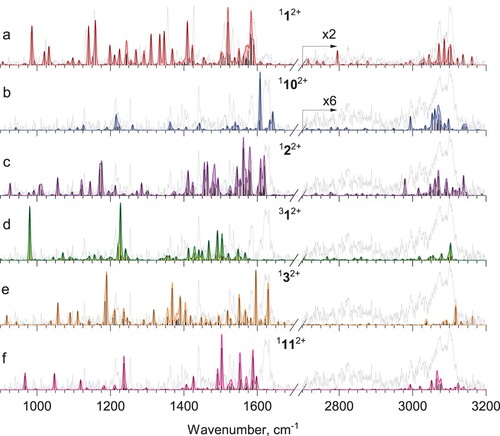
Figure 2. a) Far-IR tagging photodissociation spectrum of He-Phen2+; b) calculated vibrational spectrum of phenanthrene dication 112+; c) Vis-IR hole-burning tagging photodissociation spectrum of He-Phen2+, recorded with Vis excitation fixed at 570 nm; d) calculated vibrational spectrum of isomer 1102+ (see Figure ).
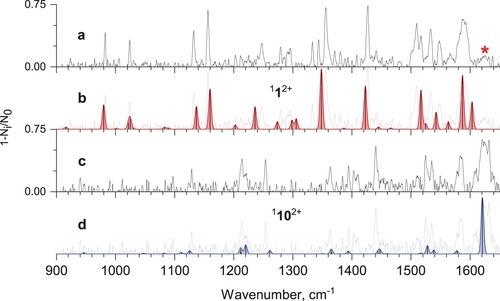
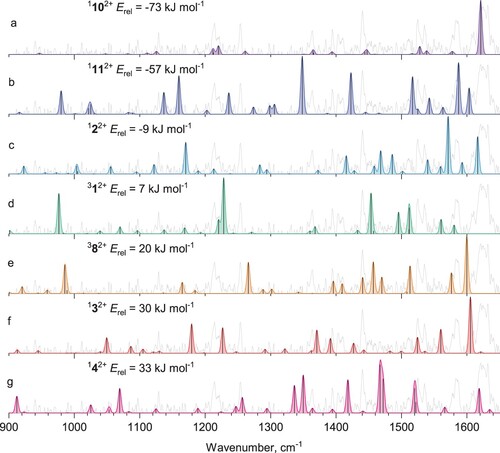
Figure 3. a) Mid-IR tagging photodissociation spectrum of He-Phen2+; b) calculated vibrational spectrum of phenanthrene dication 112+; c) IR-IR hole-burning tagging photodissociation spectrum of He-Phen2+, recorded with pump IR excitation fixed at 1427 cm−1; d) calculated vibrational spectrum of isomer 1102+ (see Figure ).
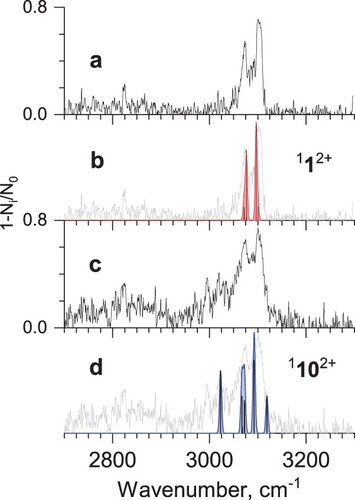
Figure 4. Geometries and relative energies in kJ mol−1 of various C14H102+ isomers. The energies are given at 0 K (including zero-point vibrational energy) relative to the energy of phenanthrene dication in the singlet ground state (112+). Note that 1252+ and 1262+ have -C≡C- motif between the phenyl rings appearing as one long connection and 1282+ has -C≡C- as a part of the macrocycle.