Figures & data
Table 1. Spore killer systems and their main characteristics
Figure 1. General mechanism and observable phenotype of spore killing. A. Spore killing phenotype in three species of ascomycetes. A.1. N. sitophila (photo by Aaron Vogan). A.2. S. pombe (photo by Nicole Nuckolls). A.3. P. anserina (photo by S. Lorena Ament-Velasquez). Killer spores are indicated by black arrowheads and killed spores by red arrowheads, if visible. Notice that in S. pombe and N. sitophila, the aborted spores are small and colorless, whereas when spore killing is inflicted by Spok genes in P. anserina they disappear completely. B. Mechanism of spore killing in ascomycetes with monokaryotic or dikaryotic spores (see Glossary). Red arrows show the killing direction. Lightning bolts represent the killing action being effective on sensitive spores, whereas the green shield represents the killing action being ineffective on resistant spores. In zygotes heterozygous at the killing locus, sensitive spores (S) are eliminated while resistant spores (R) survive. In species with dikaryotic spores (e.g., P. anserina), killing only occurs among homoallelic spores resulting from first-division segregation (FDS), whereas heteroallelic spores resulting from second-division segregation (SDS) are protected.
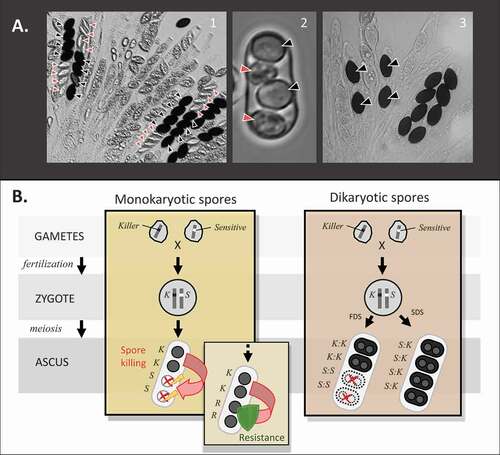
Figure 2. Genome architecture of spore killers. Within a genome, represented by a schematic chromosome (drawn as a gray bar with black centromere), spore killers (symbol: skull and bones) are found as either single genes with both the antidote and poison functions (most known instances, e.g., Spok1, Spok2, and Spk-1) or as multigene systems within nonrecombining regions (Sk-2 and Sk-3). In some cases, the spore killer genes are in low copy number and can be associated with TEs (e.g., Spok1, Spok2) or not (e.g., Spk-1). In the case of the wtf gene family, TE-associated mobilization and other processes result in the proliferation of multiple copies, including those with only a functional antidote or that are fully pseudogenized. As a special case, the Spok3 and Spok4 genes are only found within a large TE (Enterprise), which relocates them during transposition.
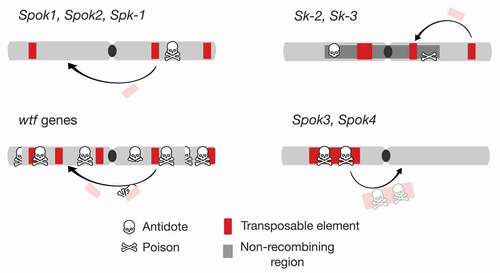
Figure 3. Mechanisms of resistance. The phenotype of resistance (partially or completely avoiding spore killing) can occur either due to the action of specific genetic elements (suppressors) or to general genome defense mechanisms. All known suppressors are homologous to their corresponding spore killer elements, although in principle nonhomologous suppressors could also evolve. In turn, homologous suppressors could be restricted to the antidote function and thus unable to drive, or be drivers themselves, creating asymmetrical killing relationships. Some known examples are given. Within fungi, two genome defense mechanisms are known to deactivate meiotic drivers: MSUD (e.g., working against Spk-1) and RIP (e.g., as hinted by the pseudogenized SpokΨ1 gene).
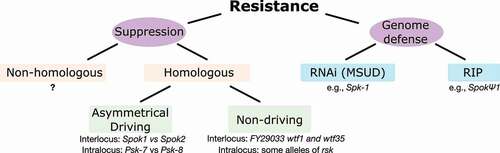
Figure 4. Models of spore killing effects on reproductive isolation. Starting from two idealized populations that diverge in allopatry, spore killers can have different effects on the buildup of reproductive barriers. A. A spore killer invades a population and a genomic conflict ensues. Along with suppressors and enhancers, other mutations can evolve and increase in frequency, acting as Bateson-Dobzhansky-Muller (BDM) incompatibilities if the invaded population comes into contact with a naive population. As a result, the hybrid crosses have additional incompatibilities on top of spore killing. B. Both populations accumulate different spore killers. Upon hybridization, the resulting spores are killed at various degrees depending on linkage between the spore killers, which lowers hybrid fitness and creates a reproductive barrier. C. Finally, a spore killer accumulates in one population, and like in A, it causes spore killing in the hybrid crosses. However, in this scenario, the spore killer eventually invades the naive population, lowering average divergence and hence reducing potential incompatibilities between the populations.
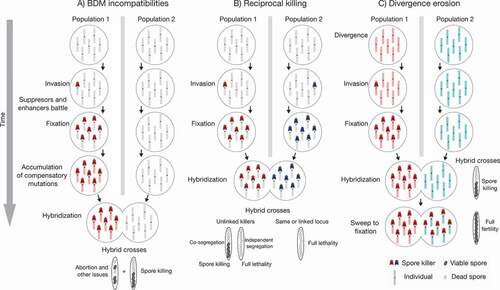
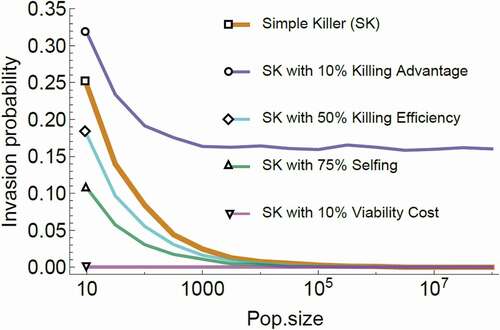
Figure 5. Simulation of the invasion probability of a spore killer as a function of population size.