Figures & data
Table 1. Demographics and baseline characteristics in the open-label titration phase (safety population) and the double-blind treatment phase.
Table 2. NRS of average daily pain intensity in double-blind treatment phase.*
Figure 3. Mean (± SE) of weekly change from baseline in NRS pain intensity in double-blind treatment phase, observed cases only (ITT efficacy population; patients at one site excluded). ITT: Intent-to-treat; NRS: Numerical rating scale.
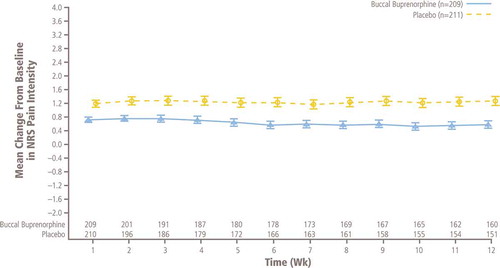
Figure 4. Sensitivity analyses of change from baseline to week 12 in NRS pain intensity. BOCF: Baseline observation carried forward; LCL: Lower confidence limit; LOCF: Last observation carried forward; MMRM: Mixed-effects model repeated measures; NRS: Numerical rating scale; PP: Per-protocol; SOCF: Screening observation carried forward; UCL: Upper confidence limit. *Missing values due to study discontinuation were imputed before the analysis as follows: (1) using the SOCF imputation if due to AEs/tolerability, (2) using the LOCF imputation if due to lack of efficacy, (3) using the BOCF imputation if due to opioid withdrawal and (4) using multiple imputation procedures for all other types of missing values. The SOCF, BOCF and LOCF imputations were implemented before multiple imputations. The sample size re-estimation was done at interim analysis and no change was made on sample size. Therefore, the unadjusted p value, estimate of treatment difference and its corresponding 95% two-sided CI were reported for the final primary analysis.
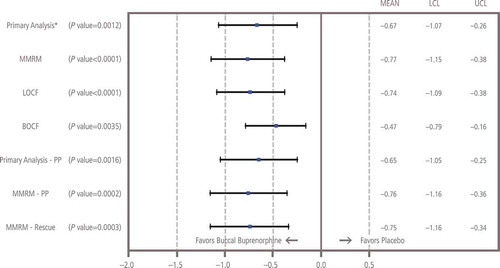
Figure 5. Proportion of responders with selected percentage pain reduction from before open-label titration to week 12 of the double-blind treatment phase.
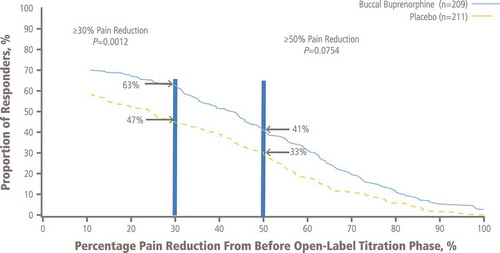
Table 3. Patient-reported outcomes in double-blind treatment phase.
Table 4. AEs during the open-label titration and double-blind treatment phases.
Table 5. Treatment-related AEs occurring in ≥2 patients treated with buccal buprenorphine during the double-blind phase.