Figures & data
Table 1. HEDIS criteria for individualized HbA1c targets.
Figure 1. Design of the randomized, open-label, parallel group, real-world, pragmatic Achieve Control study.
1Available Patient Support Program for GLA-300.2Available Patient Support Program for GLA-100 and insulin detemir.ADA: American Diabetes Association; BG: blood glucose; DPP-4: dipeptidyl peptidase-4; FPG: fasting plasma glucose; GLP-1 RA: glucagon-like peptide-1 receptor agonist; HbA1c: glycated hemoglobin; OAD: oral antidiabetes drug; PRO: patient-reported outcome; PSP: patient-support program; Q: quarter; QD: once daily; SGLT-2: sodium-dependent glucose cotransporter-2; SU: sulfonylureas; TZD: thiazolidinedione; T2D: type 2 diabetes.
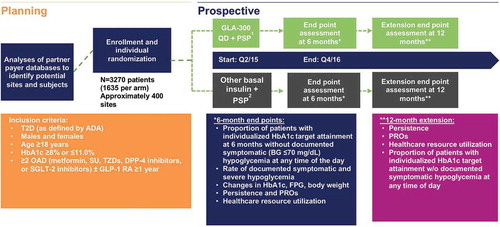
Figure 2. Major outcomes measures and timing of their ascertainment.
*Defined as a symptomatic event with documented BG ≤70 mg/dL at any time of day.**Defined as a symptomatic event with documented BG <54 mg/dL at any time of day.HbA1c: glycated hemoglobin; BG: blood glucose; FPG: fasting plasma glucose; HEDIS: Health Effectiveness Data and Information Set.
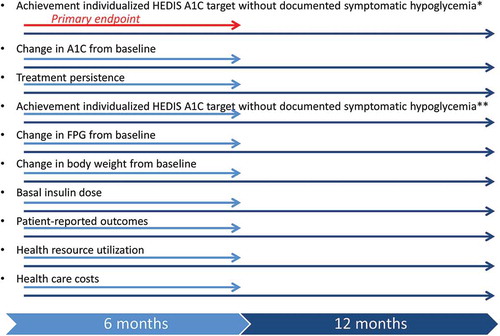
Table 2. Patient-reported outcomes instruments.
Table 3. ADA definitions of hypoglycemia in diabetes [Citation37].
