Figures & data
Figure 1. Conceptual Framework of the Observational Studies and Randomized Clinical Trial in the ER/LA Opioid Postmarketing Study Program.
DPS, doctor/pharmacy shopping; ER/LA, extended-release/long-acting; OUD, opioid-use disorder; PRISM, Psychiatric Research Interview for Substance and Mental Disorders, Fifth Edition, Opioid Study Version.
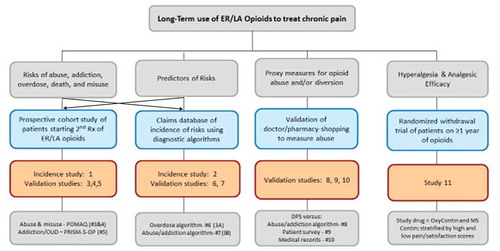
Table 1. List of Studies in the ER/LA Opioid Postmarketing Study Program, their Goals and ClinicalTrials.gov Number.
Figure 2. Conceptual Framework for ER/LA Opioid Observational Studies Linking Goals to Measurement Tools, Design, Study Sites/Databases and Validation of Measurement Tools.
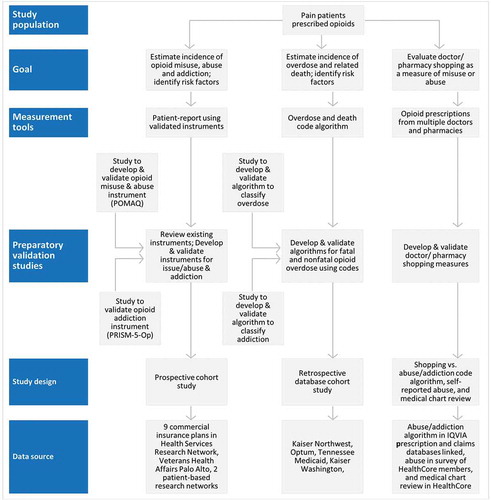
Table 2. Summary of Study Design, Data Sources, Population and Outcome Measures for 10 Postmarketing Safety Requirement Studies.
