Figures & data
Figure 1. The ADA and AACE/ACE 2020 recommendations for GLP-1RA therapy in the treatment of T2D
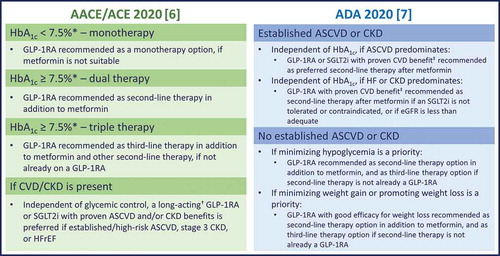
Table 1. Key efficacy endpoints for PIONEER trials 1–5, 7, and 8
Figure 2. Summary of practical guidance for initiating patients on oral semaglutide [Citation12]
![Figure 2. Summary of practical guidance for initiating patients on oral semaglutide [Citation12]](/cms/asset/6adb75b6-73b3-44a9-9403-f63e0b579444/ipgm_a_1788340_f0002_c.jpg)
