Figures & data
Table 1. Summary of PIONEER 1–8 study design and clinical efficacy results
Figure 1. Key considerations for the use of oral semaglutide across a spectrum of clinical scenarios in type 2 diabetes
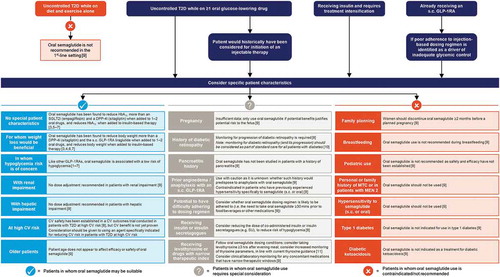
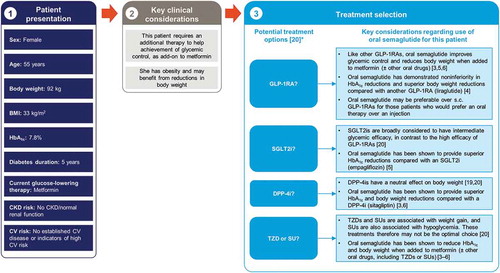
Figure 2. The rationale for oral semaglutide early in the type 2 diabetes disease course, in a patient with inadequate glycemic control on metformin: an illustrative case study
Panel 1. Estimand use in the PIONEER trial program
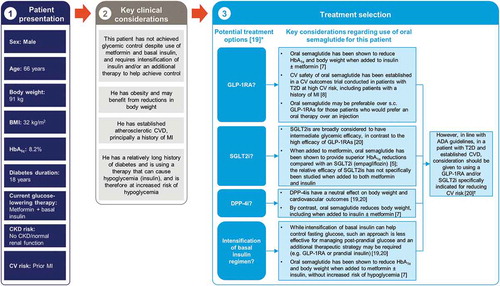
Figure 3. The rationale for oral semaglutide at a later stage in the type 2 diabetes disease course, in a patient with inadequate glycemic control on insulin and metformin and with prior cardiovascular disease: an illustrative case study
Panel 2. Exploring the risks of thyroid C-cell tumors with GLP-1RAs
Figure 4. Key communication points for counseling patients suitable for initiation of oral semaglutide [Citation9,Citation59,Citation76]
![Figure 4. Key communication points for counseling patients suitable for initiation of oral semaglutide [Citation9,Citation59,Citation76]](/cms/asset/153d9529-2c6d-44ce-9cd2-631eca415e39/ipgm_a_1798162_f0004_c.jpg)
Panel 3. An illustrative clinical discussion exploring administration recommendations for oral semaglutide
Panel 4. Key safety outcomes across the global PIONEER trial program [Citation1–7,Citation9]
