Figures & data
Figure 1. A summary of factors to consider for use of glucose-lowering medications in patients with cardiovascular disease, renal impairment, and in older patients. Adapted from [Citation5,Citation15,Citation16]
![Figure 1. A summary of factors to consider for use of glucose-lowering medications in patients with cardiovascular disease, renal impairment, and in older patients. Adapted from [Citation5,Citation15,Citation16]](/cms/asset/06ffd33a-9f18-4a1b-b262-88f0119f06ea/ipgm_a_1800286_f0001_c.jpg)
Table 1. Overview of PIONEER 5 and PIONEER 6 trial designs [Citation25,Citation26]
Figure 2. Composite primary outcome in PIONEER 6 [Citation25]
![Figure 2. Composite primary outcome in PIONEER 6 [Citation25]](/cms/asset/b9d95ee2-d826-4e3a-9208-762a17aaa8af/ipgm_a_1800286_f0002_b.gif)
Figure 3. Incidence of adverse events during PIONEER 5 and 6 (on-treatment data) [Citation25,Citation26]
![Figure 3. Incidence of adverse events during PIONEER 5 and 6 (on-treatment data) [Citation25,Citation26]](/cms/asset/a552b190-50a1-4a21-a275-fa3e25c8bdc5/ipgm_a_1800286_f0003_c.jpg)
Table 2. Change from baseline in HbA1c by age subgroup in select PIONEER studies comparing oral semaglutide 14 mg with placebo or an active comparator [Citation27]
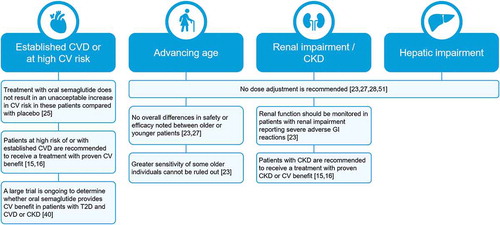
Figure 4. Summary of key clinical implications for use of oral semaglutide in patients with comorbidities. CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; GI, gastrointestinal