Figures & data
Figure 1. Study designs of SAMURAI, SPARTAN, and GLADIATOR
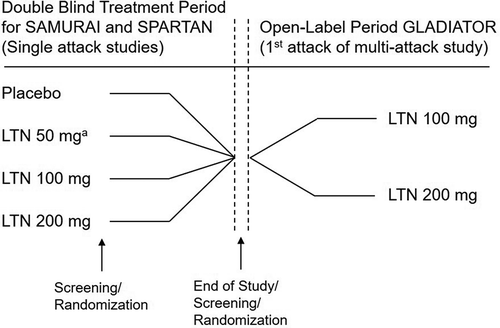
Table 1. Baseline patient characteristics and demographics
Figure 2. Efficacy outcomes for the first dose and subsequent dose
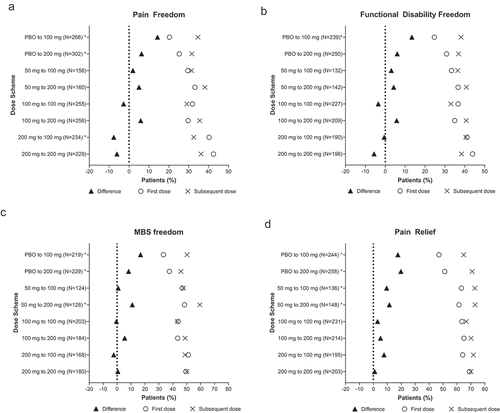
Figure 3. Incidence of patients who experienced at least one MC- TEAE with the first dose and subsequent dose
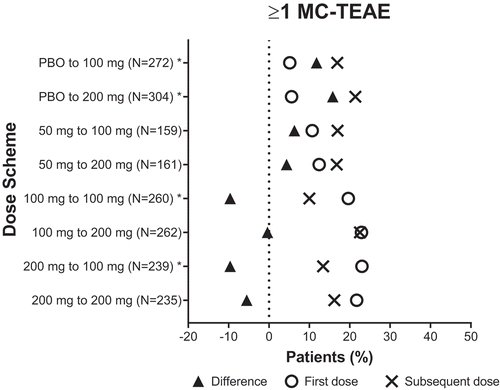
Table 2. Efficacy-TEAE balance change from first dose to subsequent dose
Table 3. Pain freedom (a), Disability freedom (b), Most bothersome symptom freedom (c), Pain relief (d), and Most common treatment-emergent adverse event MC-TEAE freedom (e) – Distribution of patients in each of four outcome shift categories by dose scheme
Figure 4. Incidence of pain-free patients after the subsequent dose among patients who were not pain-free after the initial dose (a); incidence of patients not MC-TEAE free after the subsequent dose among patients who were MC-TEAE free after the initial dose (b)
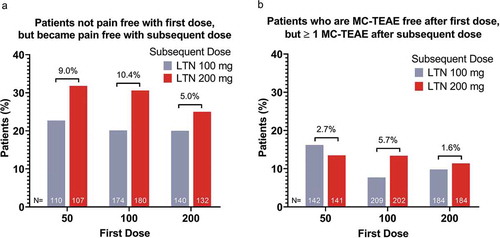
Figure 5. Effect of changing lasmiditan dose on pain response based on pain outcome after the first treated attack
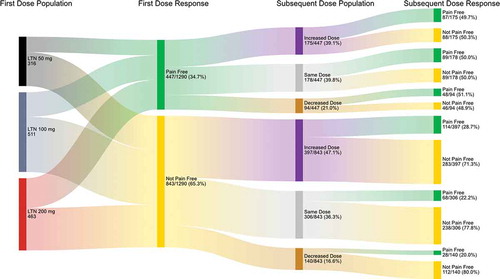
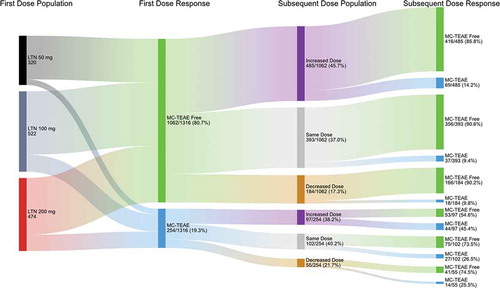
Figure 6. Effect of changing lasmiditan dose on the percentage of patients experiencing a MC-TEAE based on whether a MC-TEAE was experienced with treatment of the first attack
Data availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
