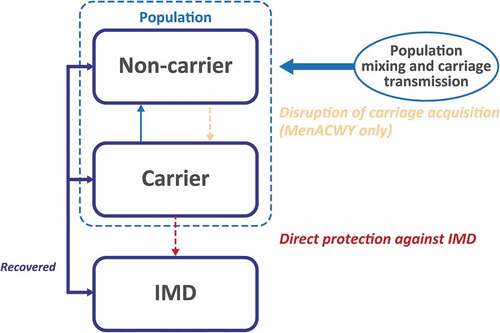
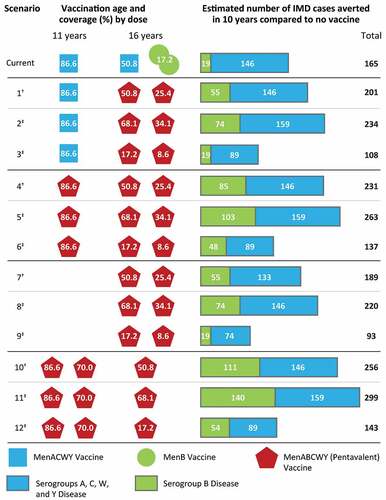
Figures & data
Table 1. IMD incidence by serogroup and age.*
Table 2. Vaccine efficacy assumptions.*