Figures & data
Exploring the Wider Benefits of Semaglutide
Figure 1. Tools used to assess quality of life outcomes in the STEP trial program.
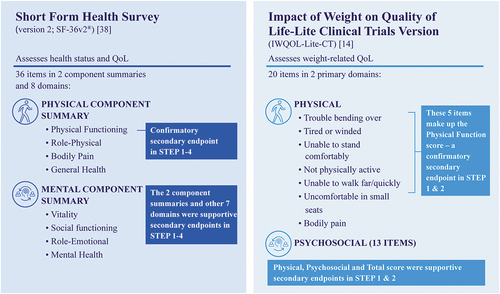
Figure 2. Effect of semaglutide versus placebo on IWQOL-Lite-CT Physical Function scores in STEP 1 and 2 [Citation31,Citation34].
![Figure 2. Effect of semaglutide versus placebo on IWQOL-Lite-CT Physical Function scores in STEP 1 and 2 [Citation31,Citation34].](/cms/asset/65ae78cd-7428-4c11-982b-d0e78de3f977/ipgm_a_2150006_f0002_c.jpg)
Figure 3. Effect of semaglutide versus placebo on SF-36v2 Physical Functioning scores in STEP 1–4 [Citation31–34].
![Figure 3. Effect of semaglutide versus placebo on SF-36v2 Physical Functioning scores in STEP 1–4 [Citation31–34].](/cms/asset/26d481ad-4f6a-4b57-87d2-af91aa810b58/ipgm_a_2150006_f0003_c.jpg)
Figure 4. Effect of semaglutide and placebo on body composition from baseline to week 68 in STEP 1 [Citation34,Citation42].
![Figure 4. Effect of semaglutide and placebo on body composition from baseline to week 68 in STEP 1 [Citation34,Citation42].](/cms/asset/56bc4661-6e90-45bd-8e48-e93ea21d77c3/ipgm_a_2150006_f0004_c.jpg)
