Figures & data
Figure 1. Meta-analysis: MSS LS mean difference of BNO 1016 treatment vs. placebo in the full analysis data set (FAS). MSS LS mean difference at day 14 is depicted for the overall pooled population of ARhiSi-1 (placebo and 480 mg BNO 1016 treatment arm) and ARhiSi-2 as well as for the subgroups with moderate/severe (MSS ≥10) and none/mild symptoms at baseline. MSS LS mean difference from the ARhiSi-2 trial is shown as reference. MSS: Major Symptom Score.
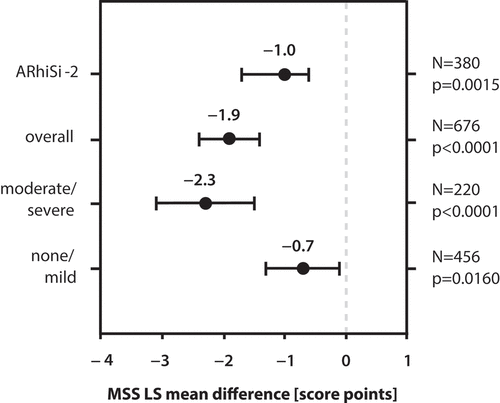
Table 1. Meta-analysis: Mean MSS and mean SNOT-20 as well as least squares (LS) mean differences at day 14 between BNO 1016 and placebo, full analysis data set (FAS).
Figure 2. Retrospective cohort study: Selection of study patients from the IMS® Disease Analyzer database.
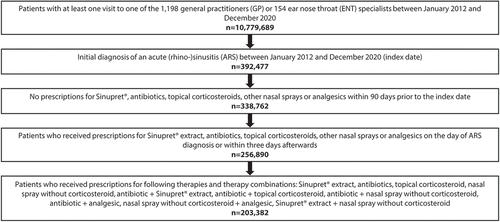
Table 2. Retrospective cohort study: Baseline characteristics of the study patients.
Figure 3. Retrospective cohort study: Prescription of antibiotics due to ARS within 31–365 days after start of therapy. Multivariable Cox proportional hazard regression adjusted for sex, age, insurance status, month and CCI. AB: Antibiotics; CI: Confidence interval; CS: Corticosteroids; INCS: Intranasal corticosteroids.
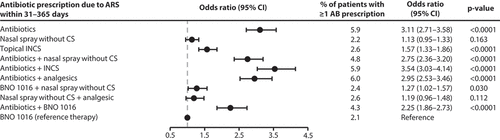
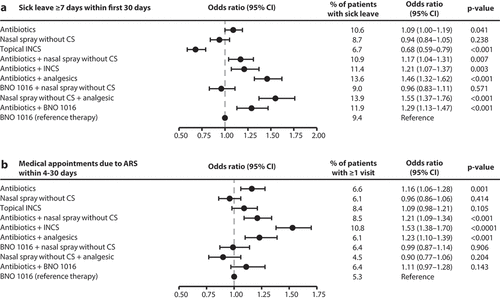
Figure 4. Retrospective cohort study: Sick leave ≥7 days within first 30 days after therapy begin (a) and medical appointments due to ARS within 4–30 days after therapy begin (b). For (a) and (b): Multivariable logistic regression; All adjusted for sex, age, insurance status, month and CCI. CI: Confidence interval; CS: Corticosteroids; INCS: Intranasal corticosteroids.
Supplemental Material
Download Zip (50.7 KB)Data availability statement
The data that support the findings of this study are available from the corresponding author, C Bachert, upon reasonable request.
