Figures & data
Figure 2. Evolution of drug delivery technologies. Characteristics of example short-, intermediate-, and long-acting stimulants used in the treatment of ADHD [Citation7,Citation18,Citation19]. Approximate timing of new technology introduction follows from left to right across the diagram. aTiming of dosing depends on whether the medication is being used as a single dose or in multiple doses and alone or in conjunction with a long-acting medication (either to provide rapid onset in the morning or to extend duration in the later part of the day). ADHD, attention-deficit/hyperactivity disorder; CR, controlled release; DR/ER-MPH, delayed-release and extended-release methylphenidate; ER, extended release; IR, immediate release; MEROS, methylphenidate extended-release oral suspension; OROS, osmotic-release oral system; SODAS, spheroidal oral drug absorption system.
![Figure 2. Evolution of drug delivery technologies. Characteristics of example short-, intermediate-, and long-acting stimulants used in the treatment of ADHD [Citation7,Citation18,Citation19]. Approximate timing of new technology introduction follows from left to right across the diagram. aTiming of dosing depends on whether the medication is being used as a single dose or in multiple doses and alone or in conjunction with a long-acting medication (either to provide rapid onset in the morning or to extend duration in the later part of the day). ADHD, attention-deficit/hyperactivity disorder; CR, controlled release; DR/ER-MPH, delayed-release and extended-release methylphenidate; ER, extended release; IR, immediate release; MEROS, methylphenidate extended-release oral suspension; OROS, osmotic-release oral system; SODAS, spheroidal oral drug absorption system.](/cms/asset/0fb3b687-17de-49ee-a0ed-09e9867b5f2c/ipgm_a_2370230_f0002_oc.jpg)
Figure 3. Comparison of pharmacokinetic curves for long-acting stimulants given in the morning versus evening-dosed DR/ER-MPH. Simulated plasma concentration versus time curves for single doses of (a) OROS MPH 54 mg, (b) d-MPH ER 20 mg, (c) MEROS 60 mg, and (d) DR/ER-MPH 100 mg. Dashed vertical lines indicate when the medication dose was taken. Adapted from Gomeni et al.: Model-based approach for establishing the predicted clinical response of a delayed-release and extended-release methylphenidate for the treatment of attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2020;40:350–358. Published by Wolters Kluwer Health, Inc. © Robert Gomeni, et al., 2020 licensed under Creative Commons Attribution 4.0 (CC BY). Proportions of immediate-release and extended-release methylphenidate data are from Childress et al. 2019 [Citation18]. conc, concentration; d-MPH ER, extended-release dexmethylphenidate; DR, delayed release; DR/ER-MPH, delayed-release and extended-release methylphenidate; ER, extended release; IR, immediate release; MEROS, methylphenidate extended-release oral suspension; MPH, methylphenidate; OROS, osmotic-release oral system.
![Figure 3. Comparison of pharmacokinetic curves for long-acting stimulants given in the morning versus evening-dosed DR/ER-MPH. Simulated plasma concentration versus time curves for single doses of (a) OROS MPH 54 mg, (b) d-MPH ER 20 mg, (c) MEROS 60 mg, and (d) DR/ER-MPH 100 mg. Dashed vertical lines indicate when the medication dose was taken. Adapted from Gomeni et al.: Model-based approach for establishing the predicted clinical response of a delayed-release and extended-release methylphenidate for the treatment of attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2020;40:350–358. Published by Wolters Kluwer Health, Inc. © Robert Gomeni, et al., 2020 licensed under Creative Commons Attribution 4.0 (CC BY). Proportions of immediate-release and extended-release methylphenidate data are from Childress et al. 2019 [Citation18]. conc, concentration; d-MPH ER, extended-release dexmethylphenidate; DR, delayed release; DR/ER-MPH, delayed-release and extended-release methylphenidate; ER, extended release; IR, immediate release; MEROS, methylphenidate extended-release oral suspension; MPH, methylphenidate; OROS, osmotic-release oral system.](/cms/asset/3b190ab3-7fdc-4cdb-8e0b-b75b3fb6c029/ipgm_a_2370230_f0003_oc.jpg)
Figure 4. Achievement of norm-referenced thresholds for impairment before and after DR/ER‑MPH dose optimization. Proportion of patients who met thresholds for impairment on the BSFQ and PREMB-R PM at baseline and after 6 weeks of DR/ER-MPH treatment in the phase 3, dose-optimization clinical trial (Study 1) [Citation33]. BSFQ, Before School Functioning Questionnaire; DR/ER-MPH, delayed-release and extended-release methylphenidate; PREMB-R PM, Parent Rating of Evening and Morning Behavior-Revised, Evening subscale.
![Figure 4. Achievement of norm-referenced thresholds for impairment before and after DR/ER‑MPH dose optimization. Proportion of patients who met thresholds for impairment on the BSFQ and PREMB-R PM at baseline and after 6 weeks of DR/ER-MPH treatment in the phase 3, dose-optimization clinical trial (Study 1) [Citation33]. BSFQ, Before School Functioning Questionnaire; DR/ER-MPH, delayed-release and extended-release methylphenidate; PREMB-R PM, Parent Rating of Evening and Morning Behavior-Revised, Evening subscale.](/cms/asset/4bb5847d-cd2f-49ee-8e95-bb963248f6a3/ipgm_a_2370230_f0004_oc.jpg)
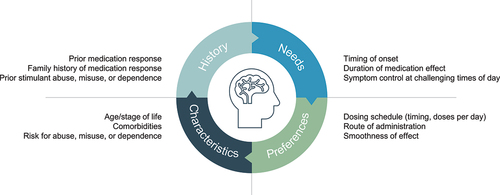
Table 1. Criteria for considering DR/ER-MPH.