Figures & data
Figure 1. Halogenated acyclic monoterpenes isolated from four Plocamium species: 1–4 from P. cornutum (South Africa; Afolayan et al., Citation2009); 5–8 from P. corallorhiza (South Africa, Mann et al., Citation2007; Afolayan et al., Citation2009,) 9, 10 from P. brasiliense (Brazil; Vasconcelos et al., Citation2010); 11 from P. cartilagineum (Portugal, Abreu and Galindro, Citation1996); 12–14 from P. corallorhiza (South Africa, Mann et al., Citation2007) and 15–19 from P. cartilagineum (California; Mynderse and Faulkner, Citation1975).
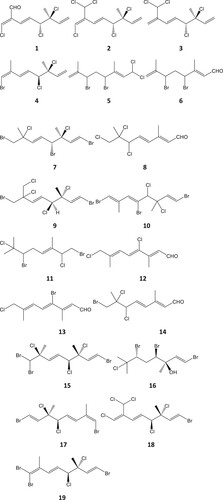
Table 1. Group rate constants used for addition of O3 to alkenes and conjugated dienes and trienes.
Table 2. Substituent factors used for addition of O3 to alkenes and conjugated dienes and trienes.
Table 3. Summary of compounds assessed and their estimated rate coefficients and lifetimes based on an OH radical concentration of 1 × 106 molecule cm−3 and an ozone concentration of 30 ppbv (∼ 7.5 × 1011 molecule cm−3 at the Earth’s surface).
Table 4. The table shows the contributions of OH- and O3-initiated oxidation, assuming [O3]/[.OH] = 7.5 × 105.
Table 5. Isolated yields of halogenated monoterpenes per dry mass of alga.
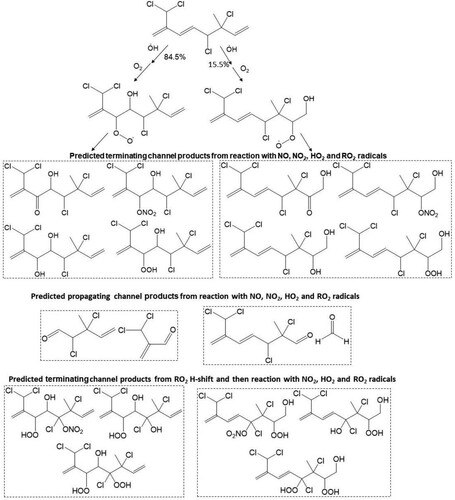
Scheme 1. Predicted major peroxy radicals, and terminating and propagating channel products from the reaction of atmospheric OH radical with the Δ3 and Δ7 olefins in 3. Both cohorts of channel products are proposed from reaction of RO2, with NO, NO2, HO2 and RO2 radicals.
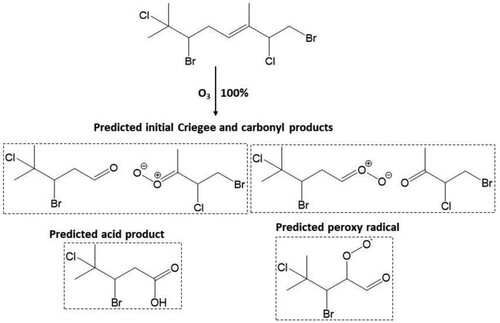
Scheme 2. Predicted major Criegee and carbonyl products, acid product and peroxy radical formed from reaction of atmospheric O3 at the Δ5 olefin in 11.
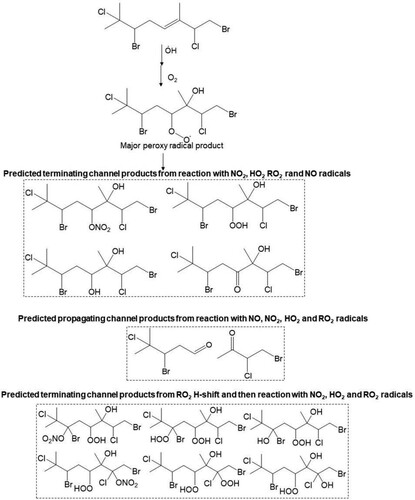
Scheme 3. Proposed major peroxy radical, and terminating and propagating channel products from the reaction of atmospheric OH radical with the Δ5 olefin in 11. Both cohorts of channel products are proposed from reaction of RO2, with NO, NO2, HO2 and RO2 radicals.
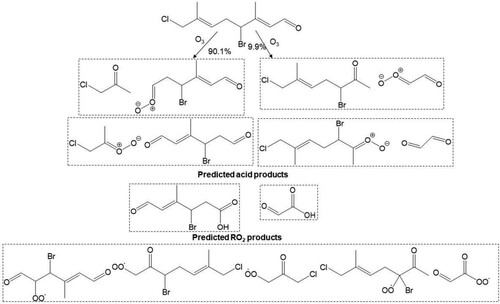
Scheme 4. Predicted major Criegee and carbonyl products, acid product and peroxy radicals formed from reaction of atmospheric O3 at the Δ2 and Δ6 olefins in 13.
Scheme 5. Predicted major peroxy radicals (RO2), and terminating and propagating channel products from the reaction of atmospheric OH radical with the Δ2, Δ6 and Δ8 olefins in 13. Both cohorts of channel products are proposed from reaction of RO2, with NO, O2, O2 and R’O2 radicals.
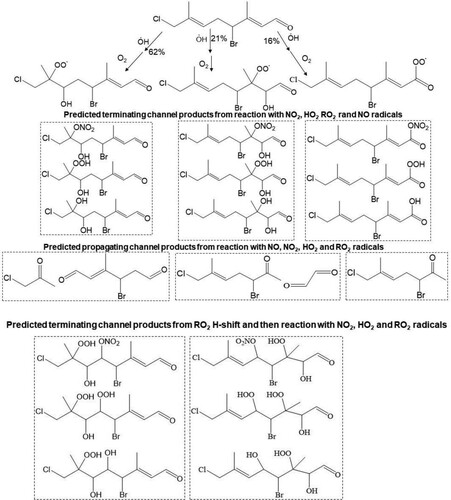
Table 6. The vapour pressure of the 1st generation OH-initiated oxidation products produced from monoterpenes 3, 11 and 13 and the percentage of gas and particle phase contribution in the formation of the SOA.
DATA AVAILABILITY STATEMENT
The methodological data will be available from the corresponding author on request.
