Figures & data
Figure 1. Flowchart showing included samples, number of samples allocated for investigation of cause of interference and number of samples analysed with alternative methods. TFT: thyroid function tests; HAMA: human anti-mouse antibodies; ASA: anti-streptavidin antibodies.
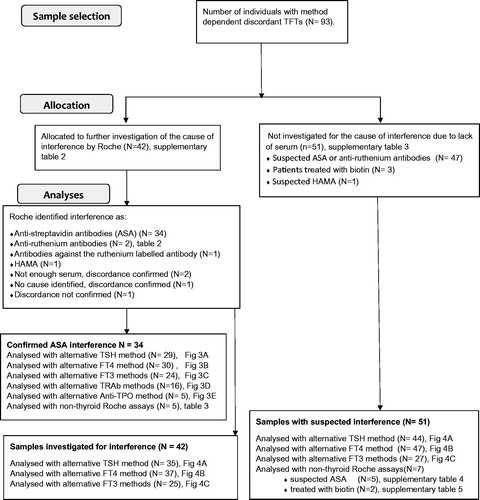
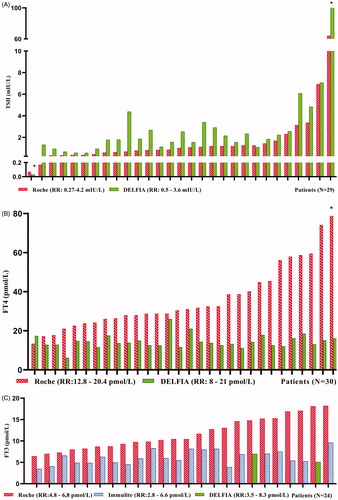
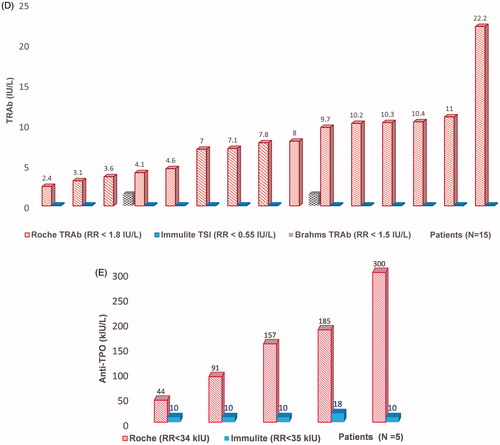
Table 1. Comparison of Roche TFT immunoassays with alternative methodology.
Figure 2. (A) Roche TSH sandwich assay, simplified. Biotin labelled monoclonal antibody from mouse against human TSH and ruthenium-labelled chimer mouse/human antibody against human TSH form an immune complex with TSH in the patient sample. Streptavidin coated paramagnetic beads (SCB) bind the biotinylated immune complex and attach to a magnet. After a washing procedure, tripropylamine (TPA) is added. TPA emits electrons. Ruthenium is excited and emits photons. The chemiluminescence signal will be proportional to the TSH level in the serum sample. (B) Possible method-dependent interference in the TSH assay. Antibodies against ruthenium or the ruthenium labelled antibody may either decrease or enhance chemiluminescent signal and lead to a false decreased or elevated TSH result. Human antibodies against mice (HAMA) may make a bridge between the two mouse-derived antibodies in the assay and thereby enhance the chemiluminescent signal and lead to a false elevated TSH result. HAMA in high titers may block the formation of an immune complex with TSH, and lead to a false reduced TSH result. Biotin treatment or endogenous antibodies against streptavidin (ASA) in the sample may prevent the binding of the biotinylated immune complex to the streptavidin coated paramagnetic beads. The decreased chemiluminescent signal will lead to a falsely reduced TSH result. (C) Roche competitive FT3 method, simplified. FT3 in the patient sample and biotin labelled FT3 analog compete for ruthenium labelled sheep antibody against human FT3. SCB bind biotin labelled FT3 analog and attach to a magnet. After a washing procedure, tripropylamine (TPA) is added. TPA emits electrons. Ruthenium is excited and emits photons. The chemiluminescence signal will be inversely proportional to the FT3 level in the serum sample. (D) Possible method-dependent interference in the FT3 assay. Antibodies against ruthenium or the ruthenium labelled antibody may either decrease or enhance chemiluminescent signal and lead to a false elevated or decreased FT3 result. Biotin treatment or endogenous ASA in the sample may prevent the binding of biotin labelled FT3 analogue to SCB. The decreased chemiluminescent signal will lead to a false elevated FT3 result.
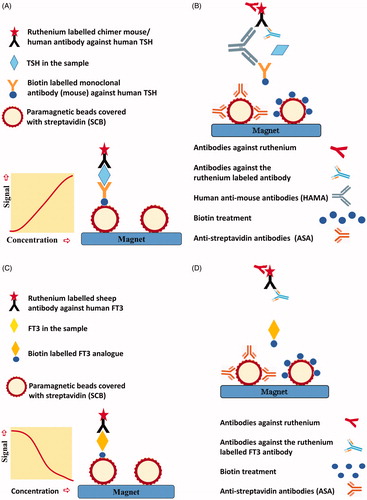
Table 2. Sera with confirmed interference to ruthenium or the ruthenium labeled FT3 antibody.
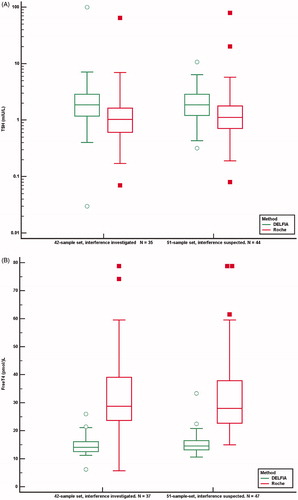
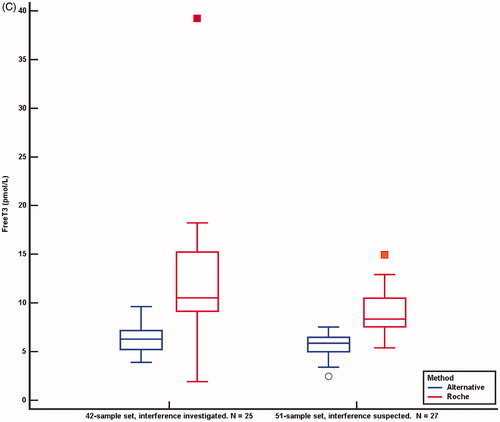
Figure 5. TSH (A), FT4 (B) and FT3 (C) concentrations analysed by the Roche method and alternative methods in 42 samples investigated for the cause of interference and in 51 samples suspected of method dependent interference. Concentrations above and below the measuring limits were given the measuring limit values. Values obtained by the Roche method were adjusted for method dependent differences according to the equations listed in . *Results above and below the measuring limit. P: patient treated with carbimazol; H: interference probably due to HAMA.
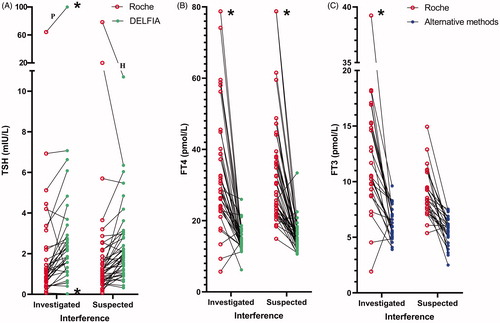
Table 3. Sera with confirmed ASA before and after incubation with SCB.