Figures & data
Figure 1. Data handling flowchart. a) Non-relevant laboratories were defined as accredited laboratories specialising in medical genetics, microbiology, toxicology, criminology, cyto-/histopathology or reproductive testing.
Figure 2. Adult total IgE reference intervals (only upper limits are shown) utilised in Scandinavia and the UK, compared to published upper reference limits (n=11 solid red points derived from 6 publications).
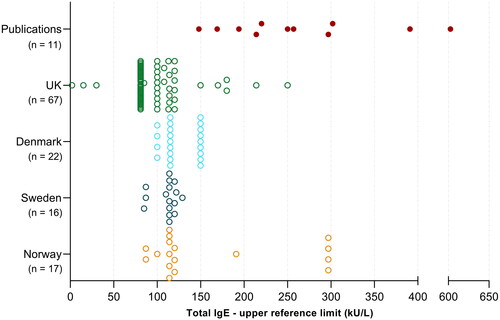
Table 1. Descriptives for acquired data sets, reference interval sources and adult total IgE upper reference limits reported by medical laboratories in Norway, Sweden, Denmark and the UK.
Figure 3. Paediatric total IgE reference interval upper limits, reported by medical laboratories (n = 122) in Scandinavia and the UK and those reported in the literature (solid red points, derived from six publications). The y-axis displays reported IgE upper reference limits on a logarithmic scale.
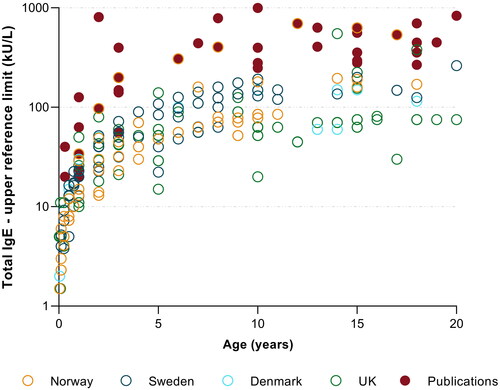
Table 2. Summary of adult total IgE reference interval studies published after 1990 utilising immunoassays, with the exception of Simoni et al. (2001) [Citation13] utilising paper radio immunosorbent test (PRIST).
Table 3. Summary of published paediatric total IgE reference interval studies performed using immunoassays identified by the literature review.
Table 4. Reference intervals provided in the package insert by market leaders of total IgE analysis in Europe with information on the international reference preparation (IRP), type of upper limit and source of data.
