Figures & data
Table 1. Baseline characteristics (according to availability of data).
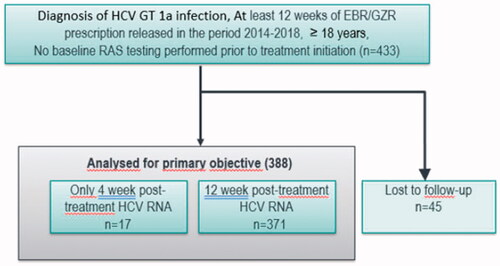
Figure 2. Outcome among 388 patients included in the mITT population and treated for 12 weeks of EBR/GZR without ribavirin and with no baseline RAS testing.
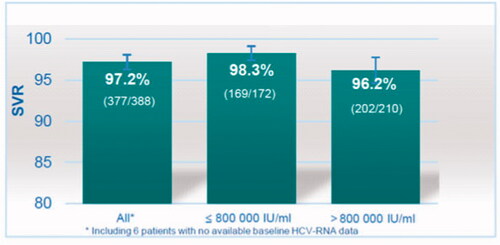
Table 2. Sustained viral response (SVR). HCV RNA results at week 12, or at week 4 to 11 if not available at week 12. Outcomes for all 433 patients together, and for 427 patients apportioned to baseline viral load above, and at or below 800 000 IU/mL, respectively. The results are presented as modified Intention-To-Treat (mITT) and Intention-To-Treat (ITT) analysis with 95% confidence intervals (95% CI).
Table 3. Virologic failures. Eleven patients did not achieve SVR. Of these 5 were successfully retreated with alternative DAA-regimes, and 1 were lost to follow up. There was no information retrieved on 5 patients (N.D, not determined).